Introduction
Protein and energy are important nutrients in animal diets, and they have an important impact on carcass fat deposition, etc (Fouad and El-Senousey, 2014). The types and contents of fatty acids and amino acids in the diet affect the growth performance and meat quality of ducks (Fouad et al., 2018).
Eels can weigh up to 30 kg and are one of the main economic fish in the world (Heinsbroek, 1991; Manik et al., 2016). They are widely distributed and have a wide variety of species (Chen et al., 2003). Eels are rich in nutrients and have high utilization value (Łuczyńska et al., 2023). At present, research on eels mainly focuses on the utilization of their waste (Siriraksophon et al., 2014). Fish meal can be used as one of the raw materials for the source of animal protein required in animal feed ingredients (Carter and Hauler, 2000). It has an extremely high protein content and consists of about 20 amino acids with a high and balanced amino acid content (Sari and Wahyuni, 2021). Adding fish meal to a diet without rice bran can increase the growth rate and feed intake of ducklings (Hoai et al., 2011). Adding fish meal to a diet containing rice bran can increase the apparent digestibility of several essential amino acids, dry matter and crude protein in ducklings, make the amino acids more balanced, and improve the growth performance of ducklings (Martin, 1998).
The types and contents of volatile flavor compounds in duck meat are affected by many factors (Zhen et al., 2022). Amino acids and fatty acids are important flavor precursors in meat (Ramalingam et al., 2019). Their levels are closely related to factors such as muscle type and feed composition (Yang et al., 2006). Eel meal is known to have a higher nutritional value when used to replace part of the feed (Carter and Hauler, 2000). It contains unique seafood flavor components and abundant flavor precursors (Zhang et al., 2025). These compounds may be metabolized and deposited in the adipose tissue and muscle cells of duck meat (Arini et al., 2017). As a result, they can alter the fatty acid composition and influence protein metabolism. These changes may ultimately affect the flavor characteristics of duck meat. This flavor change may affect consumers’ acceptance of the product.
The aim of this study was to replace part of the commercial diet with eel meal in feeding Cherry Valley ducks, systematically evaluate its effects on the physicochemical quality (such as color, shear force, moisture content, cooking loss rate, etc.) of duck meat (breast and leg muscles), lipid oxidation level and volatile flavor compound composition, and explore the potential of eel meal as a functional feed ingredient to improve duck meat quality and flavor.
Materials and methods
The produced duck feed used in this experiment was a mixture of 300 kg of frozen eel carcasses, 150 kg of Korean sorghum (to reduce moisture and increase viscosity), and 50 kg of rice bran. After grinding with a carcass processor (SUN Bio, Incheon, Korea), it was dried at 180°C for 24 h. 200 one-day- old Cherry Valley ducks were evenly divided into two treatment groups. After the ducklings in the two treatment groups were fed with commercial duck feed 1 until 3 weeks of age, the control group ducks continued to be fed with feed produced by Korean company D (commercial duck feed 2), and the test group ducks were fed with a mixed feed of 50% commercial duck feed 2 and 50% processed eel feed (Produced duck feed), and sufficient feed and water were provided during the period. The experimental period was 7 weeks. After the end of the experiment, 5 ducks in each treatment group were randomly slaughtered and sent to the Muscle Biology Laboratory of Chonbuk National University for analysis. The calculated experimental diet composition is shown in Table 1.
The breasts and legs of 10 ducks were cut out, and the skin, fat and connective tissue were removed, leaving only the breast and leg muscles. The raw breast [raw brisket muscles of the control group (RBC)] and leg [raw leg muscles of the control group (RLC)] of some control groups and the raw breast [raw brisket muscles of the test group (RBT)] and leg [raw leg muscles of the test group (RLT)] of the test group were vacuum packed and boiled in a constant temperature water bath at 70°C for 1.5 h and then cooled. The cooked breast [cooked brisket muscles of the control group (CBC)] and cooked leg [cooked leg muscles of the control group (CLC)] from commercial feed were used as the control group, and the cooked breast [cooked brisket muscles of the test group (CBT)] and cooked leg [cooked leg muscles of the test group (CLT)] from eel meal mixed feed were used as the test group. All 4 groups of raw duck meat samples and 4 groups of cooked duck meat samples were vacuumed and stored in a –40°C refrigerator for testing.
Insert the electrodes of the calibrated pH meter into each group of samples to a depth of about 2 cm, obtain the data after the readings stabilize, and repeat the measurement three times for each sample (Biesek et al., 2021).
The colorimeter was calibrated with a calibration plate and the lightness (CIE L*), red/green coordinate (CIE a*) and yellow/blue Coordinate (CIE b*) of each group of samples were measured, and each sample was measured three times.
Use deionized water (DW) to calibrate the hygrometer. After calibration, take 2.5 g of each sample and place it in an aluminum pan. Start the instrument to make it work. After completion, read the moisture content data of the sample. Repeat the measurement 3 times for each sample.
Take 350 g of sample from each group and weigh them, then put them into high-temperature resistant plastic bags and cook them in a 70°C water bath. When the core temperature reaches 70°C, take out the samples immediately and cool them in 18°C running water for 30 min. Use absorbent paper to remove moisture on the surface of the cooled samples and then measure the weight of the samples. The initial weights from before and final after cooking were used to determine the cooking loss by following equation:
After calculating the cooking loss, the cooked samples were cooled for 24 h, and the muscles were cut into strips with a diameter of 0.5 inches parallel to the muscle fiber direction. Warner-Bratzler Shear Force (WBSF) of duck muscles was measured using an instrument universal testing machine (Hwang et al., 2004).
The 2-thiobarbituric acid reactive substances (TBARS) of meat were determined according to the literature (Djimsa, 2016) with slight modifications. Briefly, 3 g of minced meat was mixed with 60 μL of Butylated Hydroxytoluene and 9 mL of DW and then homogenized. The homogenate was filtered with filter paper, and 1 mL of the filtrate was mixed with 2 mL of a mixture of trichloroacetic acid and thiobarbituric acid. The test solution was mixed and incubated in a water bath at 90°C for 15 min. After incubation, the test solution was cooled to room temperature, then centrifuged at 3,000 rpm for 10 min in a centrifuge, and finally the absorbance value at a wavelength of 531 nm was measured. Malondialdehyde (MDA; mg/kg)=absorbance×5.88 (K constant).
According to the method described with some modifications (Hoa et al., 2023), 2 g of minced meat sample and 1.0 μL of an internal standard (2-methyl-3-heptanone at 0.816 mg/mL in methanol) were placed in a 20 mL headspace vial. The extraction of aroma volatiles was done using a 75 μm SPME assembly of CAR/PDMS fiber (black, autosampler type, Supelco, Bellefonte, PA, USA) connected to a SPME auto-sampler (model: PAL RSI 85, Agilent, Santa Clara, CA, USA) of gas chromatography (model: 8890 GC system) and mass spectrophotometry (5977B MS, Agilent) at 60°C for 50 min, and then desorbed at 250°C for 5 min. The compounds were separated on a HP-5MS UI capillary column (30 m×0.25 mm i.d.×0.25 μm, Agilent) with helium as carrier gas. After being kept at 40°C for 5 min, the oven temperature was increased to 250°C at 8°C/min and kept at this temperature for 5 min. The capillary direct interface temperature was set to 250°C, the scanning mass range was set to 30–500 amu, and the scanning rate was 5.27 scans/sec. The retention index (RI) of volatile organic compounds (VOCs) was calculated using n-alkanes C7–C40 (purity ≥99%; Sigma-Aldrich, St. Louis, MO, USA) as external references. VOCs were identified by comparing retention time and RI with the GC-MS library. Semi-quantification was performed using the peak area of 2-methyl-3-heptanone of known concentration.
The odor activity value (OAV) represents the contribution of a volatile compound to flavor and is calculated by dividing the concentration of the volatile compound by its odor threshold in water (Tan et al., 2022). The odor thresholds of these compounds are from some literature (van Gemert, 2011).
All data were analyzed using generalized linear models in IBM SPSS version 24 software (SPSS Institute, IBM, Chicago, IL, USA). Multifactor-multivariate analysis of variance (M-MANOVA) was performed with feed type, muscle type, and processing method considered as fixed factors, and meat quality traits, lipid oxidation levels, and volatile flavor compounds considered as dependent variables. The significance level was set at p<0.05. Figures and tables were generated using EXCEL (Microsoft, Redmond, WA, USA) and MetaboAnalyst (www.metaboanalyst.ca).
Results and discussion
The quality traits of breast and leg muscles of ducks fed the two diets are shown in Table 2. The pH values of breast and leg muscle samples in the treated groups were not significantly different from those in the control group. Studies have shown that dietary treatment does not affect the pH value of muscle (Gariglio et al., 2021), but the pH values of RLC and RLT are higher than those of RBC and RBT, which may be because the breast muscle contains more white muscle fibers (type II) and has a stronger ability to produce acid after slaughter, while the leg muscle contains more red muscle fibers (type I; Klont et al., 1998). The CIE L* of RBT is slightly higher than that of RBC, and similarly, the CIE L* of RLT is also slightly higher than that of RBC. The CIE a* and CIE b* showed opposite differences among the four groups, but the differences were not significant. There were no significant differences in CIE L*, CIE a*, and CIE b* of the four groups of cooked meat samples, indicating that the addition of eel meal to the feed slightly affected the brightness of raw duck meat. The cooking loss of RBT is greater than that of RBC, and that of RLT is less than that of RLC. The effect of eel meal on the juiciness of duck meat varied with different parts of the duck carcass. The shear force of CBT was less than that of CBC, and eel meal improved the tenderness of sous vide duck breast, while RLT was slightly greater than RLC, and CLT was greater than CLC, indicating that it had a negative impact on the tenderness of duck legs. In terms of moisture content, CBC was higher than CBT, RLC and RLT were higher than RBC and RBT, and there was no significant difference between the other groups of samples (Pieterse et al., 2013). The MDA content of RBT, CBT and CLT was higher than that of the control group, and the lipid oxidation degree of RLC and RLT was significantly (p< 0.05) lower than that of RBC and RBT (Gong et al., 2010). Eel meal is rich in long-chain n-3 polyunsaturated fatty acids (PUFAs), such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA; Oku et al., 2009). These fatty acids ingested by ducks can be directly absorbed and used for muscle synthesis (Zhang et al., 2023). The increase in the content of unsaturated fatty acids in duck meat may accelerate lipid oxidation, which is similar to the effect of fish meal added to the diet on pork (Coronado et al., 2002). The reason why the degree of oxidation of raw duck leg muscle is usually lower than that of breast meat may be that the slow-twitch muscle fibers in duck legs are usually rich in antioxidants, and the water content of raw duck legs is relatively high, which helps to reduce the degree of lipid oxidation.
When the p-value of the interaction term among feed type, muscle type and processing method is less than 0.05, it indicates that there is a significant interaction effect among the three factors.
RBC, raw brisket muscles of the control group; RBT, raw brisket muscles of the test group; RLC, raw leg muscles of the control group; RLT, raw leg muscles of the test group; CBC, cooked brisket muscles of the control group; CBT, cooked brisket muscles of the test group; CLC, cooked leg muscles of the control group; CLT, cooked leg muscles of the test group; pH, power of hydrogen; WBSF, Warner-Bratzler shear force; TBARS, thiobarbituric acid reactive substances.
Through mass spectrometry retrieval and comparison with the RI values of normal alkanes, a total of 71 volatile flavor substances were identified in 8 groups of duck meat samples. Table 3 lists in detail the names, contents and significance (p-value) of the interactions among these factors. Among them, there are 4 acids, 11 alcohols, 30 aldehydes, 10 alkanes, 11 furanones, 3 esters, and 2 other flavor substances. It can be seen that the volatile flavor substances of duck meat are mainly alcohols, aldehydes and alkanes.
When the p-value of the interaction term among feed type, muscle type and processing method is less than 0.05, it indicates that there is a significant interaction effect among the three factors.
RBC, raw brisket muscles of the control group; RBT, raw brisket muscles of the test group; RLC, raw leg muscles of the control group; RLT, raw leg muscles of the test group; CBC, cooked brisket muscles of the control group; CBT, cooked brisket muscles of the test group; CLC, cooked leg muscles of the control group; CLT, cooked leg muscles of the test group; ND, not detected.
A total of 61 and 57 aroma compounds were identified in the test and control samples, respectively, mainly including aldehydes and alcohols, which are mainly products of lipid oxidation and partial amino acid degradation reactions (Yang et al., 2017). RBT has 3 more alcohols and 2 more aldehydes than RBC. RLT identified more types of volatile flavor substances of alcohol, aldehyde, alkane and ester than RLC. The content of aldehyde volatile flavor substances in raw duck meat in the test group was higher than that in the control group. The content of aldehyde volatile flavor substances in raw duck breast reached 38.6%, which is similar to the results of other duck meat flavor studies (Duan et al., 2023). Duck meat tissue contains small molecular natural flavor substances such as nucleotides, which can maintain their original flavor characteristics when eaten fresh (Zhen et al., 2022). Diet can affect the composition of amino acids and fatty acids in duck meat (Liu et al., 2018). Eel is rich in polyunsaturated fatty acids, which are deposited in the muscle as feed, resulting in an increase in double bonds in the muscle and increased lipid oxidation (Channon et al., 2007), thereby increasing the aldehyde flavor of duck meat.
A total of 60 volatile flavor substances were identified in cooked duck meat, among which aldehyde substances were the most abundant, accounting for more than 50% of the volatile flavor substances in cooked duck legs. CBT contains 51 volatile flavor substances, 11 more than CBC. And the total content of flavor substances in CBT is significantly higher than that in CBC (p<0.05). CLT has two more volatile flavor compounds than CLC, but the total concentration of flavor compounds in CLT is significantly higher than that in CLC (p<0.05). Overall, the types and total contents of volatile flavor substances in the four test groups were higher than those in the control group. Vacuum low-temperature cooking of meat produced more types of volatile flavor substances (Zhang et al., 2022). The main reason is that during the heating process, the fat in duck meat decomposes to produce volatile compounds such as aldehydes, ketones, and acids, which have an important influence on flavor (Zielbauer et al., 2016); diet affects the metabolism of flavor precursors in duck meat to a certain extent (Xu et al., 2023), enriching the types of volatile flavor substances in cooked duck meat.
It is generally believed that compounds with odor activity value (OAV) ≥1 are the main contributors to the aroma (Tan et al., 2022). Fig. 1 shows 23 compounds with OAV ≥1 in different groups. The activity values of these flavor-contributing compounds in cooked meat are significantly higher than those in raw meat, indicating that the concentration of flavor compounds in duck increases during the cooking process. In raw duck breast, the OAV of valeraldehyde, hexanal, heptenal, octanal, nonanal, and (E)-2-nonenal in the test group were higher than those in the control group. Similarly, in raw duck legs, the OAV of hexanal, (E)-2-octenal, nonanal, and decenal in the test group were higher than the control group, while the OAV of 1-octen-3-ol was lower than that in the control group. In the cooked duck breast, the OAV of 1-octanol, hexanal, octanal, dodecanal and 2-pentylfuran were higher than the control group, and the OAV of the other 9 flavor substances were lower than the control group. The OAV of 6 flavor substances of CLT were higher than CLC. Overall, the flavor of the test group was stronger than that of the control group.
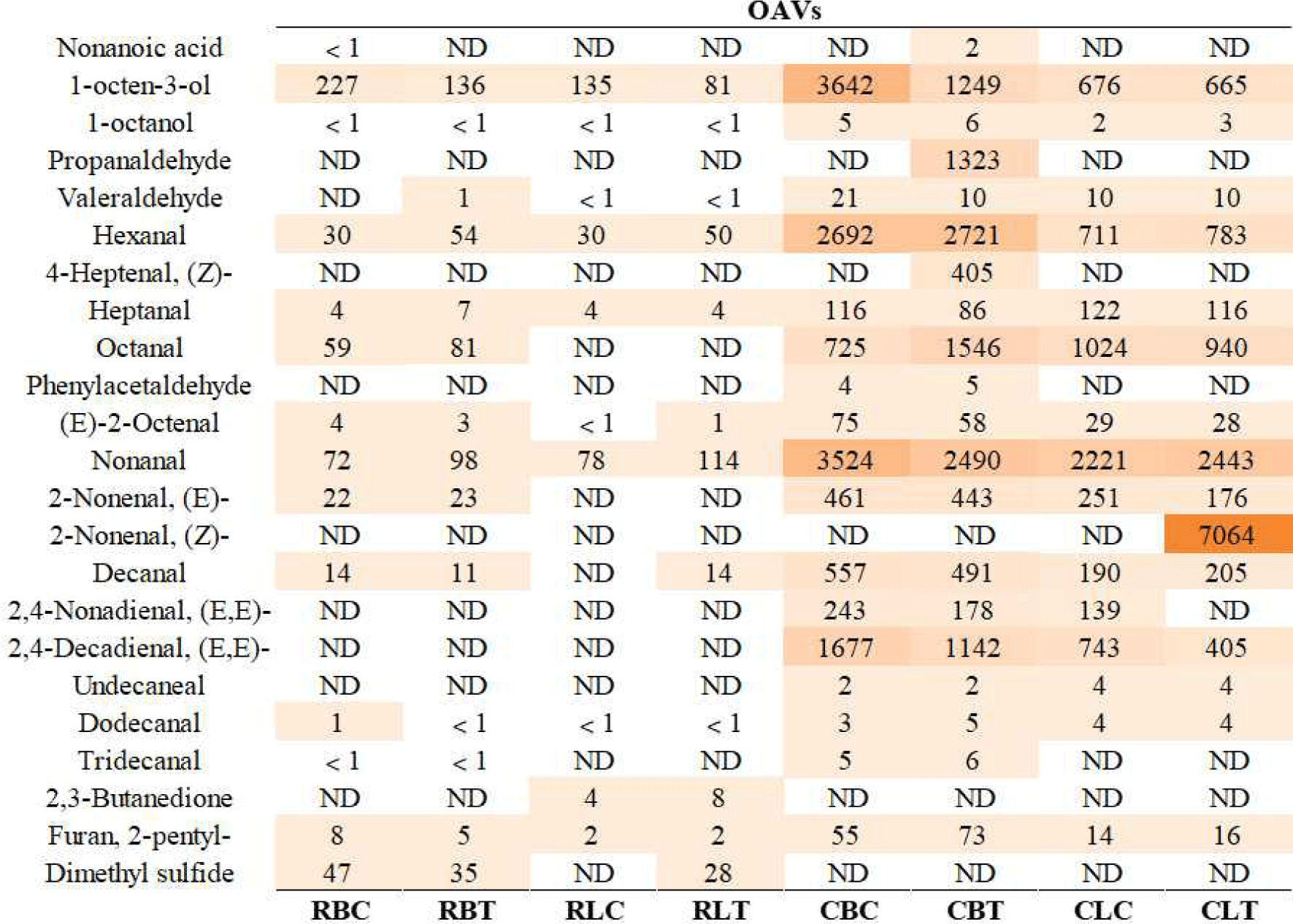
Hexanal is the most abundant secondary lipid oxidation product (Drumm and Spanier, 1991) and has a typical grassy, green and fatty taste. The nonanal content of CLT was higher than CLC. Higher nonanal levels were detected in the RBT and RLT than RBC and RLC. Nonanal has floral and citrus flavors in volatile flavors. CBT had the most alkane compounds. However, more alkane compounds were detected in RLT than RLC. Ketones can be produced in a variety of ways, and aliphatic ketones may be the products of lipid oxidation degradation (Ames and Macleod, 1984). 2-Heptanone has a strong sweet and fruity aroma. The duck leg meat in the test group contained more 2-heptanone. 2-Pentylfuran has green bean, metallic and vegetable aromas (Fors, 1983).
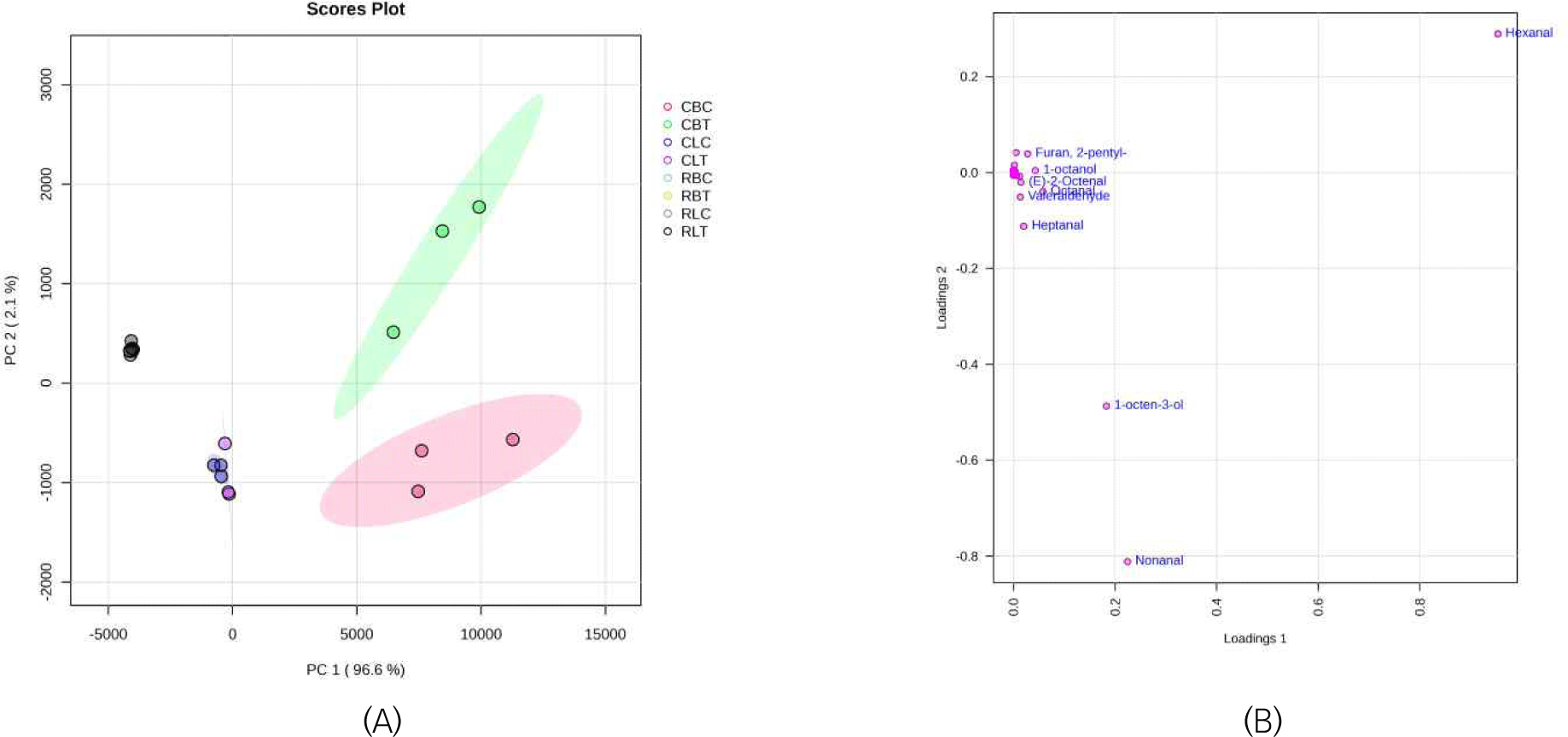
Fig. 2 shows the PCA score graphs of 8 groups of duck meat samples and the loading graph. The PCA score graph is shown in Fig 2A, where principal component 1 (PC1) explains 96.6% of the total variance and is the main distinguishing factor. Although principal component 2 (PC2) only explains 2.1% of the variance, the contribution is small, but it can still assist in distinguishing samples. CBT and CBC are very close on PC1, but are significantly separated on the PC2 dimension, and they are clearly distinguished from other groups (such as RBC, RLC, RLT, etc.). Other samples are densely clustered, indicating that their intra-group differences are small, which shows that eel meal feed and cooking have a significant effect on the key aroma substances of duck breast. From the PCA loading diagram in Fig. 2B, it can be seen that 1-octen-3-ol, Hexanal and Nonanal are far from the origin. In particular, Hexanal is in the upper right quadrant where PC1 and PC2 all are positive, indicating that the variable has a positive contribution on both principal components, and it may be the main reason for separating samples in the PC1 direction. It highlights its important role in forming the key characteristic flavor of duck meat. The results of PCA show that some volatile flavor substances can characterize the effects of feed type, cut type and processing on duck meat flavor.
Conclusion
There was no significant difference in the effect of replacing some commercial feeds with eel meal on meat quality traits, but it could significantly (p<0.05) increase the lipid oxidation degree of raw and cooked duck breasts and cooked duck legs. The volatile aroma components of duck meat are mainly composed of acids, alcohols, aldehydes, alkanes, ketones and furans, among which 1-octen-3-ol, hexanal and nonanal are characteristic flavor substances. The types and contents of aldehyde aroma components in the test group samples were more than those in the control group, and duck meat produced more types of volatile flavor substances after cooking. Eel meal contains a high level of unsaturated fatty acids, which can promote lipid oxidation after deposition in duck meat. Compounds such as aldehydes will be produced during the oxidation process, affecting the flavor components of duck meat. This study provides theoretical support for the high-value utilization of aquatic by-products in poultry farming and provides a technical reference for improving the flavor of duck meat.







