Introduction
Tuna is a popular seafood dish consumed worldwide. Tuna and tuna-like species have long been known as the major commodities of fisheries. Several types of tuna, such as Albacore, Bigeye tuna, Atlantic Bluefin tuna, Pacific Bluefin tuna, Southern Bluefin tuna, skipjack tuna and yellowfin tuna, exist worldwide (Majkowski, 2007). Recently, the demand for tuna has increased as people have become more health-conscious, thus leading to a surge in the prices of tuna meat and oil (Bell et al., 2015). Tuna production will increase over the next decade as the demand for tuna products increases in both developed and developing markets. Affluent Asian nations, including China, Japan, and South Korea, drive market expansion (Erauskin-Extramiana et al., 2023).
Yellowfin, bigeye, and skipjack are the three primary oceanic tuna species commonly found in the Indian Ocean. Yellowfin tuna is a fish species of major importance in seafood commerce in Sri Lanka. The major constituents of yellowfin tuna are 73.28% moisture, 1.52% crude fat, 23.18% crude protein, and 1.52% ash (Ovissipour et al., 2010). In addition, tuna has a diverse range of amino acid compositions, such as glutamic, aspartic, and lysine, ranging from 7.93% to 12.45% (Peng et al., 2013). Amino acids are crucial components of various healing processes, and a lack of these essential building blocks can impede recovery. Amino acids, such as alanine, proline, arginine, serine, isoleucine and phenylalanine, combine to form polypeptides that stimulate tissue healing and regeneration (Witte et al., 2002). Therefore, yellowfin tuna is a highly nutritious fish in Sri Lanka and is highly beneficial to human health (Nemati et al., 2017).
Both protein and lipid oxidation have major detrimental effects on fish, including tuna (Yetisen, 2021). Lipid oxidation in fish and fish products can lead to unpleasant odors and a decline in the overall quality, which can negatively impact consumer satisfaction. Moreover, lipid oxidation can alter the structural makeup of fish muscle (Baron et al., 2007). It is assumed that lipid oxidation modifies the nutritional value and quality of fish based on prior data. On the other hand, the omega-3 fatty acids found in yellowfin tuna are abundant and susceptible to oxidation due to lipid breakdown. Therefore the high amount of omega-3 fatty acids in yellowfin tuna may decrease owing to lipid oxidation resulting in a reduction in its overall nutritional value (Guizani et al., 2014).
Metabolomics is the dynamic field of analysis of small- molecule metabolites (<1 kDa) in biological systems, offering insights into biochemical pathways and cellular function, and providing a holistic view of physiological states (Johanningsmeier et al., 2016). Meat metabolic profiles linked to sensory acceptability (Antonelo et al., 2020), flavor and aroma (Aung et al., 2023), color, and oxidative stability (Ma et al., 2017) have been successfully obtained using 1D1H nuclear magnetic resource (NMR). Therefore, metabolites should be investigated to evaluate the quality of yellowfin tuna under refrigerated conditions.
With the increase in market demand, it is necessary to ensure the oxidative stability and biochemical processes of yellowfin tuna. However, not much study has been done on the biochemical processes in yellowfin tuna under different storage conditions. Therefore, this study aimed to evaluate oxidative stability under refrigerated conditions. In addition, changes in metabolites were compared between the initial and final storage days (day 7).
Materials and Methods
Yellowfin tuna were provided by Ceylon Fresh Seafood (Pvt.) Ltd, Sri Lanka. After the catching fish, they were stored under the frozen conditions, They were received to the company after one month. Then loins were separated from the yellowfin tuna fish, and their average weight was approximately 2.750± 0.250 kg. Samples were vacuum-packed and storing under frozen condition shipped to the university laboratory same day, and stored under refrigerated condition (4°C).
Fish samples (2 g) and 18 mL of distilled water (DW) were mixed and homogenized. A pH meter (PL-700PV, Gemmy Industrial Corp., Taiwan) was used to measure the pH of the filtrate after the homogenate had been filtered through the Whatman No. 4 filter paper (Whatman, Seoul, Korea). The pH was measured on days 1, 3, 5, and 7 under refrigerated conditions (4°C).
The 2,4-dinitrophenyl hydrazine (DNPH) assay was used to figure out how much protein carbonyl was in the fish sample according to (Alinasabhematabadi, 2015). A 3 g sample was combined with 30 mL of phosphate buffer (20 mM, pH 6.5 containing 0.6 M NaCl) and thoroughly homogenized. From this mixture, two aliquots of 0.2 mL each were taken for analysis. Both aliquots were treated with 1 mL ice-cold trichloroacetic acid (10% TCA) and were placed in cold water for 15 min. They were then centrifuged at 2,000×g for 30 min. After discarding the supernatant, the residue was mixed with 1 mL of TCA and the above procedure was repeated. A 0.5 mL solution of DNPH [10 mM DNPH dissolved in 2.0 M Hydrogen chloride (HCl)] was applied to one aliquot for treatment. 0.5 mL of 2.0 M HCl was used as the blank for another aliquot. The samples were covered with aluminium foil and vortexed for 1 h using a vortex machine (Model No; M 15, Vortex, Milano, Italy). The sample was mixed with 0.5 mL of ice-cold 20% TCA solution before vortexing and placed in an ice bath for 15 min. Then, 1.0 mL of ethanol/ethyl acetate (1:1, V/V) was added after centrifugation at 2,000×g for 20 min, with the supernatant being discarded. Next, the samples underwent vortexing and centrifuging at 2,000×g for 20 min. This procedure was repeated three times. The pellets were kept in a hood for 15 min following the removal of the supernatant. The pellets were dissolved in 1 mL of 6.0 M guanidine hydrochloride prepared with a 20 mM phosphate buffer at pH 6.5. This mixture was vigorously vortexed for 30 min covered with aluminum foil to protect it from light. Centrifugation was conducted to the final solution at 9,500×g for 10 min. An absorbance was measured at 280 and 370 nm on days 1, 3, 5, and 7 under refrigerated conditions (4°C). This equation was used to compute the carbonyl concentration;
The antioxidant activity of yellowfin tuna was evaluated using the (2,2-diphenyl-1-picrylhydrazyl) DPPH radical scavenging activity (Alma et al., 2003). Firstly, homogenization was performed for the combination of a 2 g sample and 18 mL DW. After filtration through Whatman No.4 paper (Whatman), 3 mL of filtrate was centrifuged at 3,000×g for 10 min. The supernatant (4 mL), DW (1.6 mL), and DPPH solution (2 mL) were mixed by vortexing and incubated in the dark at room temperature for one hour. The absorbance was measured at 517 nm on days 1, 3, 5, and 7 under refrigerated conditions (4°C). 2 mL of DW and 2 mL of methanol were combined to create a blank solution. 2 mL of the DPPH solution and 2 mL of DW were combined to create the control solution. The scavenging activity was computed with the below formula;
The protocol reported by (Kim et al., 2019) was used for the extraction of samples and NMR analysis. Firstly, 5 g of fish sample using 20 mL of 0.6 M perchloric acid was homogenized, and centrifugation was conducted to the homogenate (Continent 512R, Hanil, Daejeon, Korea) at 3,500×g for 20 min. The supernatant was centrifuged, after adjusting the pH 7.0 with Potassium hydroxide (KOH). After taking the filtrate through Whatman No. 1 filter paper (Whatman), it was lyophilized (Lyoph-Pride, LP03; Ilshin BioBase, Dongducheon, Korea). Finally, lyophilized samples were diluted in 20 mM phosphate buffer (pH 7.4) was used with D2O containing 1 mM 3-(trimethylsilyl) propionic-2,2,3,3 d4 acid (TSP). A Bruker 600 MHz cryo-NMR spectrometer (Bruker BioSpin, Rheinstetten, Germany) was used for NMR analysis, and Topspin 4.0.8 (Bruker BioSpin) was used for spectral analysis. TSP was used as an internal standard during the quantitative analysis process.
Experimental data with three replicates were analyzed using the Minitab statistical software package, version 20. One-way analysis of variance (ANOVA) analysis with a 95% confidence level was used to statistically analyze the data. MetaboAnalyst 6.0 (https://www.metaboanalyst.ca/) was used to analyze partial least squares discriminant analysis (PLS-DA) and variable important projection (VIP) score.
Results and Discussion
Changes in pH and lipid oxidation play a crucial role in meat quality during storage by influencing the oxidation of myoglobin (Chauhan and England, 2018). Fig. 1 illustrates how the pH of yellowfin tuna changes while it is being stored. Overall, the pH increased from 6.13 to 6.27 during storage (p< 0.05). After storing 3 d, the pH value decreased insignificantly but increased again after day 5 of storage (p>0.05). It is assumed that the occurrence of glycogenolysis, which causes the breakdown of glycogen into lactic acid, decreases pH in the fish tissue (Nazir and Magar, 1963). On the final storage day (day 7), the pH of the yellowfin tuna increased significantly (p<0.05). The pH is affected by inorganic compounds containing nitrogen as well as by the release and formation of inorganic phosphates. Bu et al. (2022) reported a positive correlation between pH and freshness, and that the pH of southern bluefin tuna increased during storage. the pH value of tuna fish biofluid increased after 7 d of storage owing to the production of alkaline bacterial metabolites (Fazial et al., 2017). Rodríguez et al. (2004) claimed that muscle pH rises as a result of secondary alkaline substances such as ammonia being released by endogenous and microbial enzymes encouraging protein breakdown. In general, the pH of fish is stated to be between 6.0 and 6.5 immediately after it is caught, while rotten fish have a pH above 7.0 and pH values up to 6.8 are acceptable (Jinadasa et al., 2015). Therefore, the present results show that yellowfin tuna still had an optimal pH range after 7 d of storage.

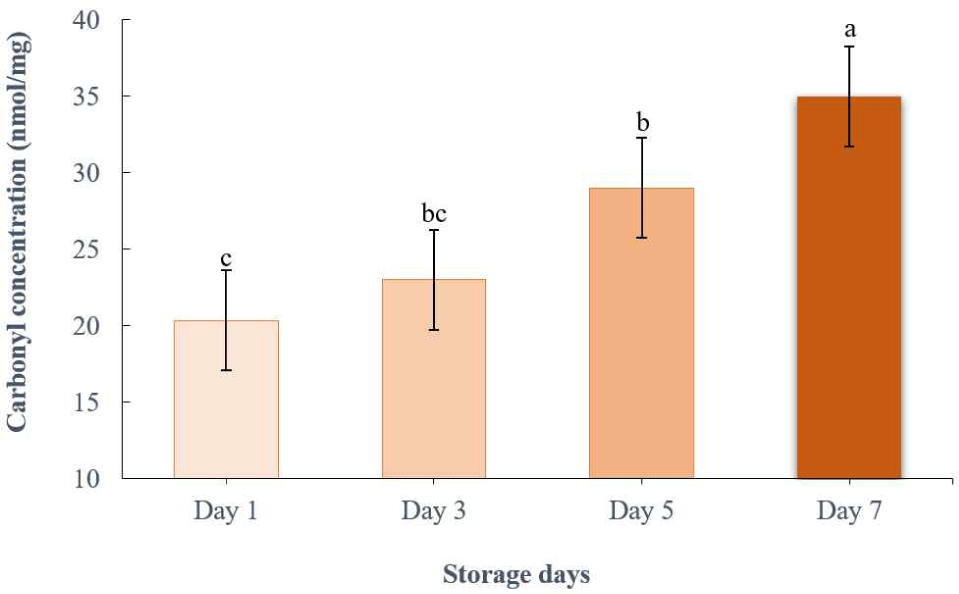
The DNPH is a common method to evaluate the total number of carbonyls in a protein, allowing for the quantification of protein oxidation (Dalle-Donne et al., 2003). In yellowfin tuna, the carbonyl content is a crucial marker of protein oxidation. Fig. 2 shows how the carbonyl content of yellowfin tuna changed over 7 d in the refrigerator. As the number of storage days increased, the carbonyl content of the yellowfin tuna increased (p<0.05). No significant contrast was between days 1 and 3, or between days 3 and 5 (p>0.05). Kjærsgård and Jessen (2004) mentioned that the increase in carbonylation was caused by high salt-soluble proteins, primarily carbonylated protein fractions. This was substantiated by an increase in the carbonyl concentration of myofibrillar protein in thin-lipped mullets after 10 d of refrigerated storage (Tokur and Polat, 2010). Protein oxidation may deteriorate the overall quality of meat products, affecting their texture and flavor (Xiong and Guo, 2020). In addition, high levels of protein oxidation can impair the nutritional value of foods, reducing the bioavailability of amino acids and resulting in the loss of essential amino acids (Domínguez et al., 2022). Therefore, there should be a great concern regarding higher protein oxidation in yellowfin tuna during long-term refrigerated storage.
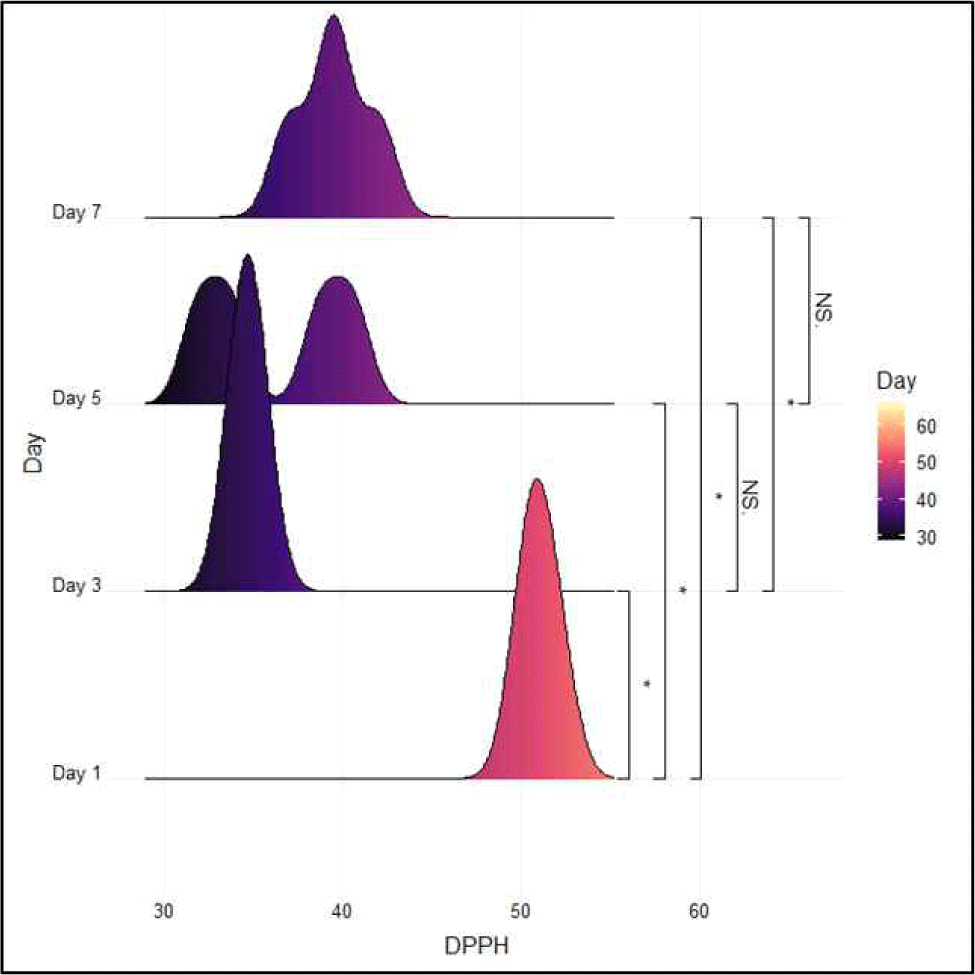
The DPPH assay determines the potential of substances to act as free radical scavengers. It is also used to determine the antioxidant capacities of fish fillets, other foods, and food items (Ceylan et al., 2019). The DPPH radical scavenging activity of yellowfin tuna is shown in Fig. 3. After 7 d of storage, the initial 51.90% DPPH scavenging activity decreased to 39.50%. The DPPH radical scavenging activity significantly decreased on day 3 (p<0.05). A decrease in DPPH levels, as observed during refrigerated fish storage, indicates an increase in the production of secondary lipid oxidation products, such as aldehydes (Kolakowska, 2002). However, there was a significant increase on days 5 and 7 (p<0.05), but with a lower value than the initial value (p<0.05). These alterations could include the activation of antioxidant mechanisms or the ingestion of prooxidants, thereby increasing the DPPH levels. DPPH radical scavenging activity may increase due to the formation of antioxidant compounds such as tryosine, creatine and lactat (Lawler et al., 2002; Torkova et al., 2015). Rest of this response of microbial activity, and activation of enzymes during refrigerated storage affect to the DPPH radical scarvenging activity. The current metabolite compound results also showed an increase in the levels of antioxidant compounds in the refrigerated yellowfin tuna.
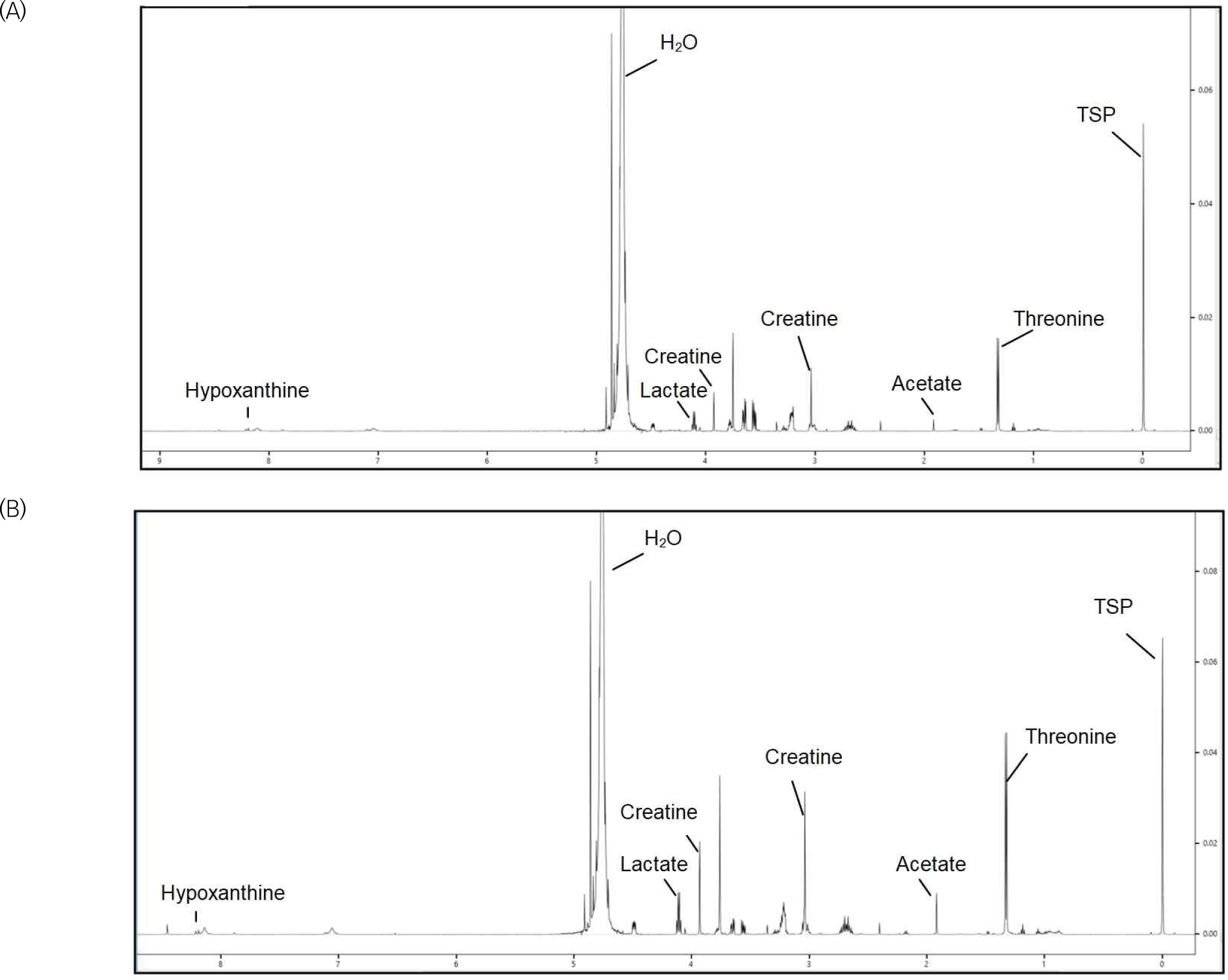
The 1D1H NMR spectra from the first and seventh days of refrigerated storage of yellowfin tuna are shown in Figs. 4A and B, respectively. The 1H NMR spectra of the yellowfin tuna muscle samples contained a few assignable amino acids, nucleotide-related metabolites, miscellaneous metabolites, and energy-related metabolites. According to the principal component analysis (PCA) biplot and PLS-DA, the metabolite compounds on days 1 and 7 are significantly different from each other geographically, with Component 1 accounting for 97.7% (Figs. 5A and B). Significant variation in group discrimination for PLS-DA is reflected in the VIP score, which displayed four metabolite compounds (threonine, lactate, tyrosine, and creatine).
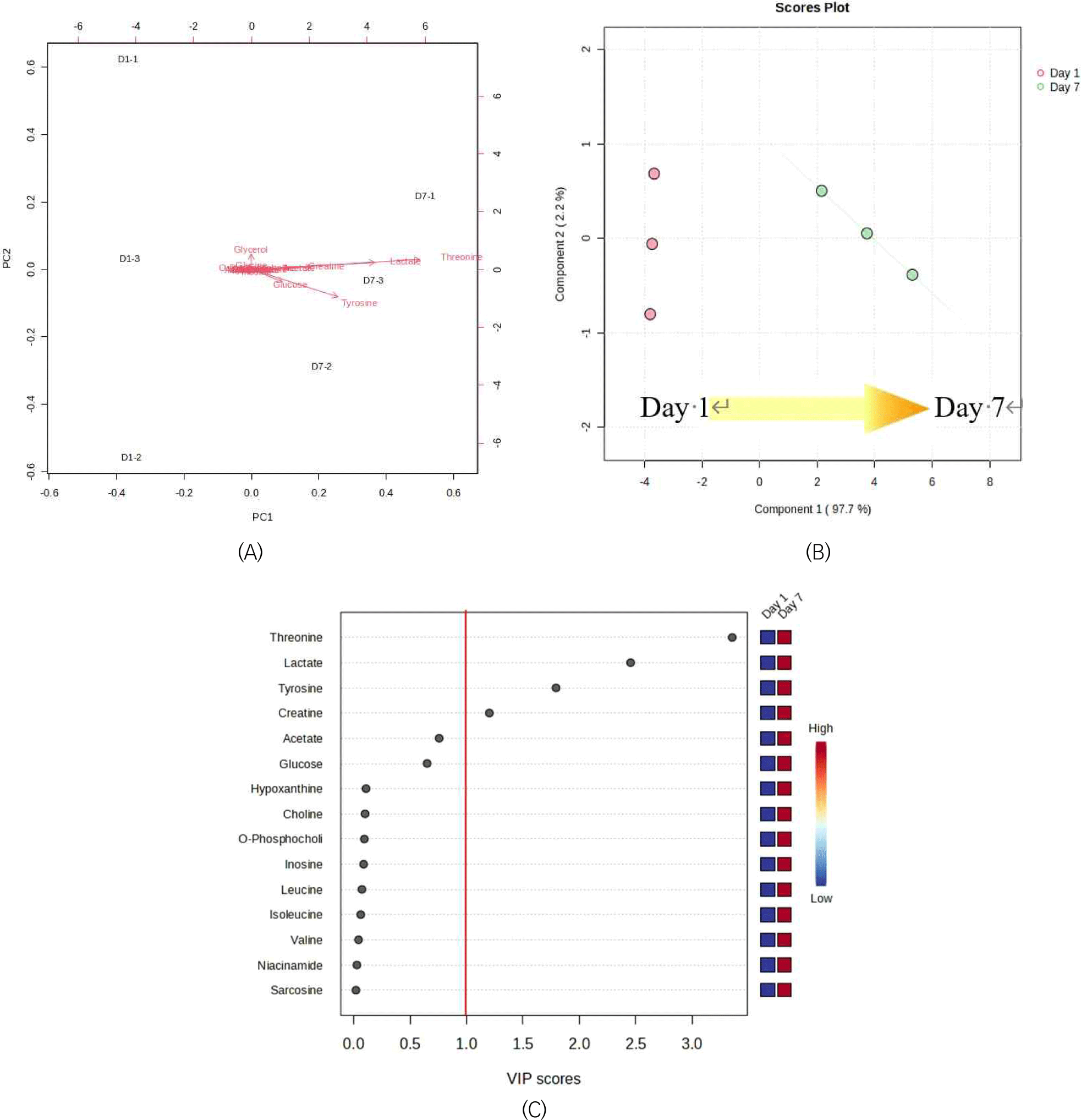
Based on 1D1H NMR spectra, 23 metabolites were identified, and the assigned compounds are listed in Table 1. During meat storage, proteins undergo various changes, including degradation. This process leads to the release of free amino acids and peptides from large protein structures. They enhance the production of savory and umami flavors in fish (Konosu and Yamaguchi, 1982) and increase fish antioxidant activity (Chan et al., 1994). According to the current research, free amino acids, including isoleucine, leucine, threonine, tyrosine, valine, and niacinamide significantly increased after 7 d of storage. Ruiz-Capillas and Moral (2001) and Shiba et al. (2014) found that muscle autolysis and microbial growth caused these amino acid changes. Özden (2005) and Ruiz-Capillas and Moral (2004) also found that maintaining fish at a specific temperature significantly increases their isoleucine content. Previous studies have revealed that isoleucine, leucine, tyrosine, and valine are linked with bitterness (Kodani et al., 2017). Li et al. (2004) and Pripp and Ardö (2007) reported that bitter peptides have certain structural properties and many bitter dipeptides exhibit angiotensin-converting-enzyme (ACE)-inhibitory action. The structural requirements for ACE-inhibitory activity are related to these properties. In addition, alanine, glycine, isoleucine, leucine, threonine, and valine are involved in protein breakdown, where bacterial metabolism of these amino acids leads to the production of ammonia, contributing to the increase in pH. Niacinamide, also known as vitamin B3 or nicotinamide, is found in various foods, including meat, fish, dairy products, and grains (Gehring, 2004). Niacinamide (nicotinamide) is a derivative of niacin (nicotinic acid) and a precursor to the coenzymes nicotinamide adenine dinucleotide and nicotinamide adenine dinucleotide phosphate (Gehring, 2004). Niacin and its derivatives indirectly contribute to antioxidant defense by supporting the function of enzymes involved in neutralizing free radicals and protecting tissues from oxidative damage (Finger et al., 2009). Therefore, increasing niacinamide levels may help protect yellowfin tuna from oxidative processes that can lead to rancidity and off-flavors.
With the use of 1H NMR, the nucleotide-related metabolites hypoxanthine and inosine were identified. Enzymes present in fish muscles gradually deplete adenosine triphosphate, converting it successively into adenosine diphosphate, and adenosine monophosphate. Subsequently, the inosine 5′-monophosphate (IMP) formed undergoes degradation into inosine, further breaking down into hypoxanthine through autolytic and microbial action (Surette et al., 1988). This hypoxanthine is further transformed into uric acid. The freshness indices are based on the ratios of nucleotide-related metabolites to adenosine triphosphate (ATP) degradation components (Karube et al., 1984). Notably, there is an inverse relationship between uric acid content and the freshness of fish meat, making it a significant analytical method for freshness evaluation (Gökoğlu and Yerlikaya, 2015). In addition, Bodin et al. (2022) reported that hypoxanthine contributes to the bitter, and off-flavors of fish. The current study found a substantial increase in hypoxanthine and inosine levels after 7 d of storage (p<0.0), likely due to bacterial activity.
Recent studies have found that choline and its derivative metabolites, phosphoethanolamine, significantly increase after 7 d of storage. It is one of the products released during the phospholipid hydrolysis (Sardenne et al., 2016). Choline is an essential nutrient that plays various crucial roles in the body and is a component of several important molecules, such as acetylcholine (a neurotransmitter), phosphatidylcholine (a major component of cell membranes), and sphingomyelin (Moretti et al., 2020). However, the production of these compounds indicates both the oxidative susceptibility and health benefits of yellowfin tuna because the ester bond between the glycerol backbone and fatty acids undergoes hydrolysis, releasing free fatty acids such as docosahexaenoic acid and eicosapentaenoic acid (Refsgaard et al., 2000).
Furthermore, 10 energy-related metabolites were collected during the refrigerated storage of yellowfin tuna. Acetate, lactate, glucose, and creatine levels significantly increased after 7 d of storage (p<0.05). Acetate, lactate, and succinate are organic acids produced by microbial metabolism, particularly in the context of muscle-based food storage (Chiou et al., 1998). Acetic acid contributes to the flavor profile of fish and may act as a preservative by creating an acidic environment that inhibits the growth of spoilage microorganisms (Bórquez et al., 1994). Lactate, which is generated from pyruvate via glycosis, is abundant in tuna muscle because of its role in burst swimming activity (Guppy and Hulbert, 1979). Succinate and lactate can reduce metmyoglobin activity, improve meat color and lower lipid oxidation (Bramstedt, 1962). Additionally, lactate is crucial for the development of pleasant scents and the degree of freshness degree (Ramanathan et al., 2011). Creatine, a type of phosphocreatine retained in muscle tissue, may contribute to the reduction of oxidative stress (Wu, 2009). In addition, an increase in the glucose levels in fish during refrigerated storage can occur because of various biochemical and microbial processes. The usual source of glucose is the stored muscle glycogen. Glucose reduces water activity by creating an osmotic environment, and (Sionek et al., 2021) found that glucose has a substantial negative correlation with pH. Therefore, high glucose concentrations, whether synthetic or endogenous, can inhibit meat deterioration (Nychas et al., 1998).
The 1D1H NMR spectra of yellowfin tuna revealed a significant increase in several metabolite components after 7 d of refrigerated storage. In addition to an increase in bitter peptides, which are beneficial to health, the levels of antioxidant compounds also increased. Although refrigerated yellowfin tuna lost some freshness, increased organic acids may inhibit microbiological growth. Furthermore, the products of phospholipid hydrolysis indicated an increase in the nutritional quality of yellowfin tuna.
Conclusion
The study found that while yellowfin tuna’s freshness decreased during 7 days of refrigerated storage, its pH remained acceptable. Antioxidant activity increased after 7 days, and protein carbonyl content increased. NMR analysis showed a increase in beneficial compounds like bitter peptides, antioxidants, and antimicrobials.







