Introduction
Cyperus esculentum is a perennial grass-like plant with spheroid tubers, pale yellow they are edible, sweet, nutty, flavoured tubers which contain adequate nutrients (Nina et al., 2020). According to Nina et al. (2019), modifying tiger nut via various processing techniques for sensorial acceptability has been opined. According to Nina et al. (2020), three varieties of tiger nuts namely: black, brown and yellow. Only yellow and brown could be readily found. However yellow cultivar is preferred for its yields of milk and functional bioactive components properties (Ndubuisi, 2009; Adejuyitan et al 2009). According to Nina et al. (2020), oil obtained from tiger tuber has better quality. Tiger nut oil could remain in a uniform liquid form at refrigeration temperature, and therefore has been opined suitable for food applications due to its high oleic acid and low polyunsaturated fatty acid (linoleic acid and linolenic acid) contents (Ezebor et al., 2005; Nina et al., 2020). The presence of polyunsaturated fatty acids and gamma-tocopherol contents in tiger nut stabilizes its oxidative tendencies. It is a quality oil and recommended for cooking compared to other oils because it resistant to chemical agent decomposition even at high temperatures (Nina et al., 2020; Shaker et al., 2009). However could be altered by visceral enzymes either in-vitro or in-vivo.
The importance of vegetable oils is increasing even as source of health enhancing compounds in which tiger nut oil has become one Nina et al. (2020). However oil stay are generally affected by chemical, physical and intrinsic agent against their shelf stay. This work seeks to investigate the quality of tiger nut oil when stored for future applications.
Materials and Methods
Brown, black and yellow tiger nuts were bought from open North bank market Makurdi, Benue State, Nigeria and identifications made at Department of Agronomy, Federal University of Agriculture, Makurdi (Fig. 1).
Normal n-hexane, a non-polar solvent according to AOAC Internatoinal (2012) method was used to extract tiger nut oil from the resulting flour. Flour samples (105.0 g sample A, 105.0 g for sample B and 105.0 g for sample C) were used for tiger nut oil extraction using soxhlet extractor (Fig. 2; Adejuyitan, 2011). The lipid was extracted for 5 h. with a 500 mL volumetric flask containing the solvent, which was heated with an electric heater at 70°C. Solvent extracts were evaporated off using rotary evaporator and later oil was oven dried at 105°C for 1 h and stored in bottles to be analysis (Fig. 3).
Moisture content was determined by the AOAC Official method, AOAC Internatilnal (2010). Dried and weighed moisture dishes were added 5 g of tiger nut oil repecively. This was heated in an oven Memmert at 105°C for 1 h, then cooled in a desiccator containing phosphorus peroxide and then weighed. This was repeated until a constant weight was obtained.
Ws=weight of moisture dish+sample
Wh=weight of moisture dish+sample after heating
Wt=weight of tare/moisture dish
The method described by Benchamaporn et al. (2009) was used. Fifty milligram oil sample was accurately weighed into a twenty-five milliliter volumetric flask and dissolved in a small volume of 1-butanol and made up to volume with 1-butanol. 0.5 mL of the oil sample was transferred to a dry test tube and 5 mL of thiobarbituric acid (TBA) reagent solution (0.2883G/100 ml of 90% glacial acetic acid) added. The test tube was closed with a ground-glass stopper, mixed thoroughly and placed in a thermostatic bath at 95°C. After 120 min, the tube was removed from the thermostatic bath and cooled under running tap water for 10 min until it reaches room temperature. The absorbance of the reaction solution was measured at 530 nm using distilled water in the reference cuvette. A blank was also prepared and read. The result was calculated using the equation below:
A=absorbance of the test solution
B=absorbance of the reagent blank
m=the weight (g) of the test sample
The AOAC (2012) method was adopted. Oil sample (2.0 g) was accurately weighed into a conical flask, and dissolved in a solvent mixture containing 12 mL chloroform and 18 ml glacial acetic acid. To the solution 0.5 ml of a saturated aqueous potassium iodide solution were added. The flask was stoppered and allowed to stand for 1 min. Thirty milliliters of water were added and the solution was titrated with 0.1 M sodium thiosulphate solution until the yellow colour gone. Starch solution (0.5 mL) was introduced and titration continued with the reagent added slowly until the blue-black colour disappeared. During titration, the flask was continuously and vigorously shaken to transfer the liberated iodine from the chloroform layer to the aqueous layer. A blank titration was also performed, and the peroxide value was obtained from the formula (Sadoudi and Ali, 2017):
PV=peroxide value
V=volume of Na2S2O2 solution used for the sample test
V0=volume of Na2S2O2 solution used for the blank test
N=normality of Na2S2O2 solution
m=weight of the oil sample taken
Into dry beaker was measured 2 g of pre-heated oil to 50°C and re-measured. Aliquots of ethanol were added to the oil to completely free the fatty acids. The ethanol-oil mixture was then titrated with 0.1N NaOH using phenolphthalein indicator. The volume (V) of NaOH required to produce the first permanent pink colour was recorded to evaluate free fatty acid (FFA) content of the oil from the formula (AOAC International, 2012).
M=relative molecular mass of palmitic acid=256
V=volume of NaOH used
N=Normality (concentration) of NaOH
m=weight of oil used
10=constant
Results and Discussion
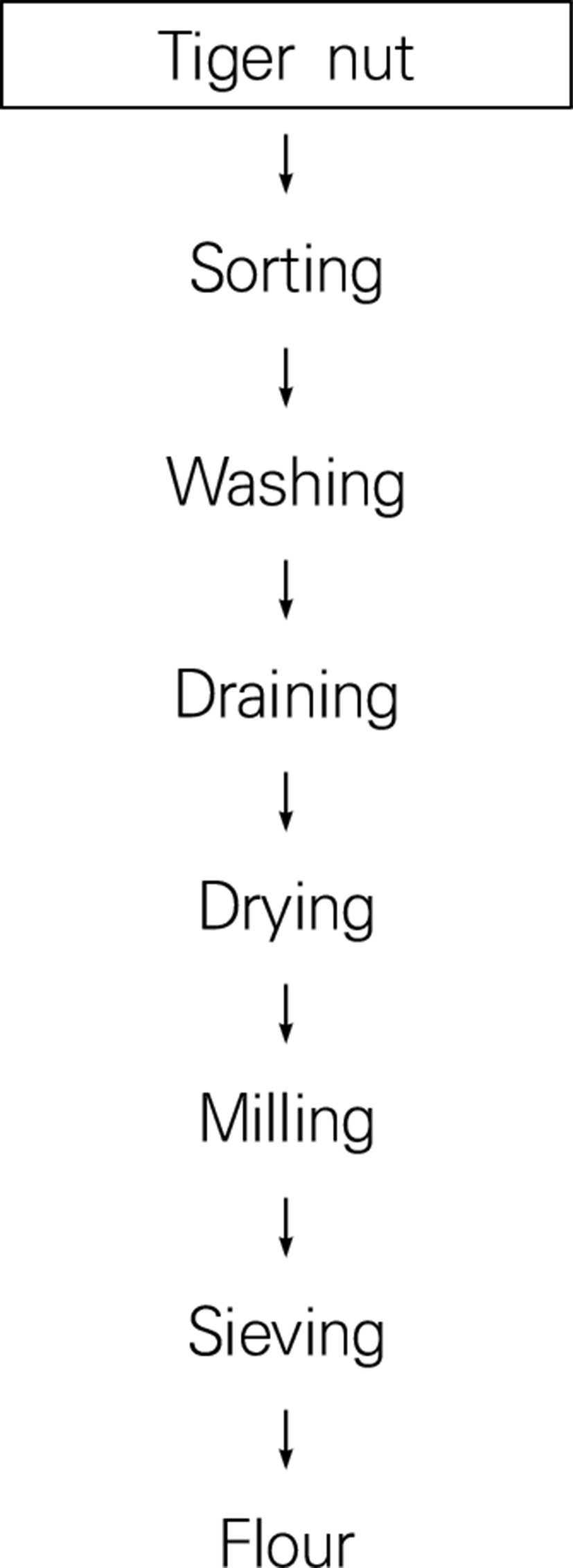
Storage time increased but the moisture content increased and decreased slightly as the storage time increased significantly at (p<0.05; Fig. 4). It was also in agreement with the acceptable limit for moisture content of oils. The moisture content of food however gives an indication of its shelf-stay and nutritive value, hence low moisture content is a requirement for long storage life (Okene and Evbuomwan, 2014).
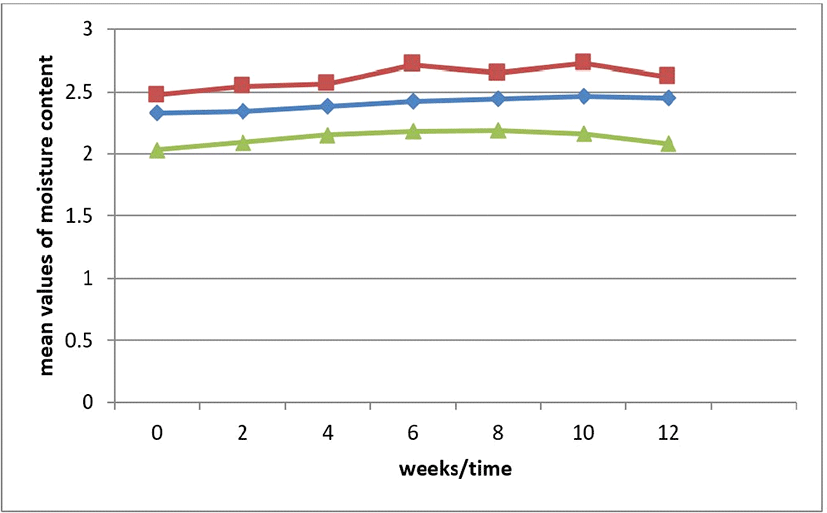
Effect of storage on FFA of tiger nut oil is shown in Fig. 5. FFA values of the stored oil for sample of oil from black tiger nut from 0 to 12 weeks were ranged between 0.75–0.87, sample Brown (0.39–0.41) while sample yellow ranged from 0.19–0.22. The FFA values for sample black and brown increased during storage while in sample Yellow; there was an unsteady decrease in FFA during storage.
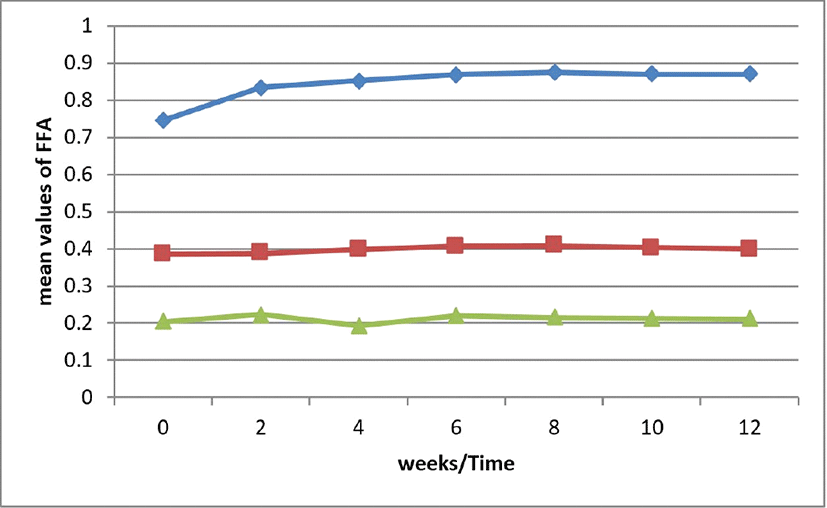
This procedure measures the malondialdehydes (MDA) formed as the split product of an endo peroxide of unsaturated fatty acids resulting from oxidation of a lipid substrate (Antolovich et al., 2002). Brown tiger nut oil seem stable from the graph. the black was stable for TBA but start decreasing with time. The yellow sample was unstable. These variation may be due to varieties of samples sources and ability to absorb transmit or deflect light.
Measures in secondary lipid oxidation products changes of the oils during storage for a period of 3 months are shown in Fig. 6. Significant (p<0.05) difference existed between black and brown and as storage time increased only for yellow, there was changes in the TBA values as the storage time increased. It seems that the increase and decrease of TBA value were as a function of time of heating depends on the number of malondialdehydes produced. This result values collaborated with Gulla and Waghray (2011) work that reported storage studies on sesame and rice bran oil. TBA measures secondary lipid oxidation products, which are also responsible for the rancid taste during storage (Decker et al., 2000).
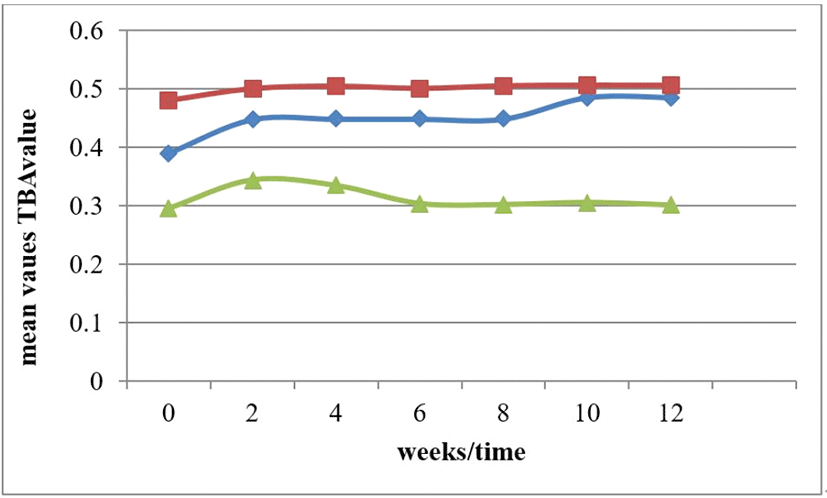
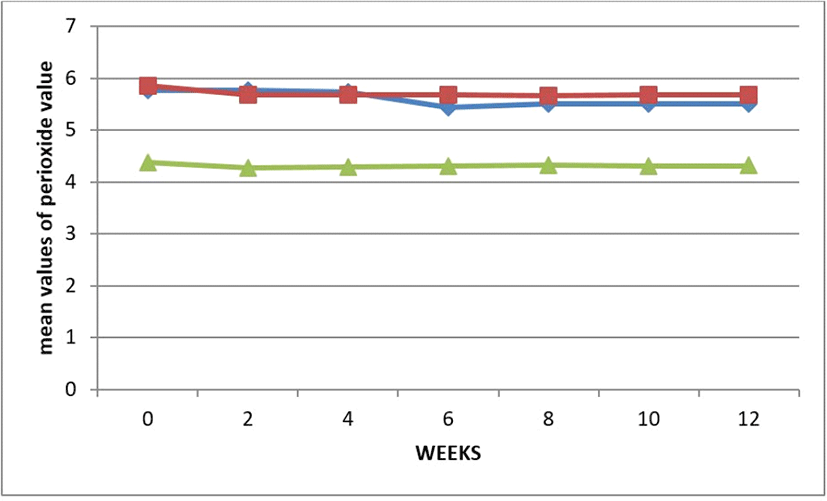
Storage time increased but the peroxide value decreased as the storage time increased. Fig. 7. The graph indicate a stable peroxidicity of the oil samples, implying that the oil could be readily stable under favourable state. The Peroxide value of an oil or fat is used as a measurement of the extent to which oxidation reactions have occurred during processing and storage. Autoxidation in this oil are relatively stable, involving less oxygen that could leads to deterioration of fats and oils which could have form off-flavors and off-odors. The values obtained during storage did not exceed the limit of 10 meq/kg (SON, 2000). According to Alhibshi et al. (2016) these varietal sample oil are safe. Peroxide values of this oils are less than 10 milliequivalents /kg Rancidity set in at peroxide value between 30 and 40 milliequivalents/kg,. However, high peroxide values are a definite indication of rancid fat, but moderate values may be the result of depletion of peroxides after reaching high concentrations (Kamsiah and Yusof, 2012).
Conclusion
Quality parameters of stored tigr nut oil showed that there was change during storage but the changes in FFAs, perioxide values, thiobabeturic acid values for all varietal samples are with recommended limits by standared hence food and home application plausible. The properties of the stored tiger nut oil for the 12 weeks showed that the oil is could be useful and can play important roles in providing food security. Because the sources are relatively cheap and abundant, these could enhance livelihoods, improve nutritional status and social wellbeing for vulnerable groups.









