Introduction
Duck meat is prized for its unique flavour and nutritional value, but its quality and flavour properties can be significantly affected by raising methods and processing techniques. As consumers’ demands for food quality increase, optimizing feed formulas and processing techniques to improve duck meat’s sensory characteristics and nutritional quality has become a research hotspot. Research in recent years has found that specific dietary ingredients can significantly affect the growth and meat quality of meat ducks. For example, as a natural pigment, red yeast rice can effectively improve meat colour and tenderness (Yudiarti et al., 2019); microecological preparations can improve immunity and delay protein degradation by regulating intestinal flora (Soumeh et al., 2021). In addition, low-temperature slow cooking, as a mild cooking method, helps to retain the original colour and texture of duck meat, and reduce protein denaturation and lipid oxidation, thereby enriching its flavour characteristics (Zhang et al., 2022).
As an important factor affecting the quality of duck meat, flavour compounds mainly include esters, aldehydes, ketones, alcohols and other volatile components. The composition and concentration of these components change under different feeds and processing conditions, affecting the meat—overall sensory experience (Mancinelli et al., 2021). During low-temperature cooking and storage, lipid oxidation, protein degradation and Maillard reaction products in duck meat will jointly affect the production of flavour compounds, such as hexanal, 2-octenal, and 1-octen-3-ol. Common flavour molecules are gradually generated to form a complex flavour profile (Xie et al., 2022).
Fishmeal is widely used in animal feeds due to its high protein content and abundance of ω-3 fatty acids, and has been shown to help improve growth performance and immune status in poultry and aquaculture species (Alagawany, 2019; Miles and Chapman, 2006). Despite these advantages of fishmeal, few studies have systematically evaluated its effects on the storage quality and flavor evolution of duck meat. In particular, its effects on lipid oxidation kinetics, protein degradation and the production of aroma-active compounds during storage remain unclear.
In addition, most of the previous studies have focused on fresh or conventionally cooked meat products, while vacuum cryogenic cooking (vacuum-sealed low-temperature prolonged cooking) is increasingly recognized for its ability to retain moisture, inhibit oxidation, and preserve volatile flavor compounds. (Dominguez-Hernandez et al., 2018; Gómez et al., 2019; Kathuria et al., 2022; Roldán et al., 2013). However, the interactions between dietary fishmeal and vacuum low-temperature cooking methods on the quality and flavor evolution of duck meat are largely unexplored.
Although conventional quality parameters such as pH, cooking loss, and thiobarbituric acid reactive substances (TBARS) provide essential information on meat spoilage and oxidation, they offer limited insights into the complex and dynamic changes of flavor compounds during storage. Advanced analytical techniques such as solid-phase microextraction–gas chromatography–mass spectrometry (SPME-GC-MS), combined with multivariate statistical methods like partial least squares- discriminant analysis (PLS-DA) and variable importance in projection (VIP) analysis, have proven effective in profiling volatile flavor compounds (Bleicher et al., 2022; Huang et al., 2005; Jin et al., 2021; Li et al., 2022). However, few studies have applied these tools to investigate storage-induced flavor changes in duck meat modulated by dietary interventions.
Therefore, the present study aimed to fill these research gaps by investigating the effects of fishmeal-added diets on the physicochemical quality, oxidative stability and volatile flavor profiles of vacuum-low-temperature-cooked duck breast meat under frozen conditions. The results are expected to provide new insights into feed formulation strategies and quality-oriented duck meat processing, and the spectral analysis of volatile flavor components will provide theoretical support for enhancing the flavor quality and market competitiveness of duck meat products.
Materials and Methods
The feed for this study was manufactured by first placing 300 kg of dead eel (Anguilla japonica) stored in a freezer, 150 kg of domestic sorghum to reduce moisture content and make it stick together, and 50 kg of rice bran in a fish carcass processor (SUN Bio, Gunpo, Korea) and crushing and drying them at 180°C for 24 h. The feed ingredients are shown in Table 1.
The study included 200 one-day-old Cherry Valley broiler ducks divided equally into control and treatment groups. The control group received commercial duck feed (young duck feed for the first three weeks, then growing duck feed) for 7 weeks. The treatment group received a 50:50 mix of commercial feed and eel mixed feed for the first three weeks, then a 50:50 mix of growing duck feed and mixed feed until week 6. Both groups had ad libitum access to feed and water. After 7 weeks of feeding, five ducks from each group were randomly selected and slaughtered. The left breast fillets (approximately 350 g each) were excised from each carcass, vacuum-packed individually in polyethylene bags, and transported under refrigeration (4±1°C) to the Muscle Biology Laboratory at Jeonbuk National University. Samples were then subdivided for physicochemical and volatile compound analyses.
For physicochemical assessments [pH, color, moisture content, shear force, volatile basic nitrogen (VBN,and TBARS), one duck breast was used per individual at each time point, with a total of five biological replicates per group (n=5). Each measurement was conducted in triplicate (technical replicates), yielding a total of N=15 measurements per parameter per group per time point.
The remaining breast samples were vacuum-packaged and frozen at –18°C until analysis. Frozen samples were thawed at 4±1°C for 24 h prior to measurement, and all evaluations were performed immediately after thawing.
For GC-MS analysis of volatile compounds, five individual duck breast samples were analyzed per group at each storage point (0, 7, 14, and 21 days). Each sample was tested once (n=5, N=5), and the mean values were used for multivariate statistical analyses (PLS-DA, VIP, heatmap).
All samples were selected using a completely randomized design to minimize sampling bias. Duck breasts were not further subdivided prior to testing, and each intact sample was treated as an independent biological replicate.
Duck breast is prepared by removing the skin, fat and connective tissue from the pectoral muscle, referring to Kathuria’s method, by vacuum packing the duck meat, cooking it at 70°C for 1.5 h, and then cooling it with running water (Kathuria et al., 2022).
pH measurements were performed using a calibrated pH meter (HI99163, Hanna Instruments, Smithfield, RI, USA). pH tests were conducted on fresh (0 days) samples and frozen samples (at various time points) after thawing and equilibration at 4°C for 30 min. The probe was inserted three times at random positions in the thickest area of each duck breast, and the reading was stabilized. Prior to measurement, the pH meter was calibrated with standard solutions (pH 4.0 and 7.0).
Flesh color was measured using a Konica Minolta CM-2500d spectrophotometer (Konica Minolta, Tokyo, Japan). Duck breast samples were thawed at 4°C for 24 h. After equilibration at 4°C for 30 min, three measurements were taken on the surface of each sample.
Duck breast samples were stripped of visible fat and connective tissue and ground at 4°C using a stainless steel laboratory grinder equipped with a 4 mm diameter disk. The ground samples were then thoroughly homogenized, and their moisture content was measured using a halogen moisture analyzer (HR73, Mettler Toledo, Greifensee, Switzerland) at 105°C. Approximately 2.5 g of sample was placed on an aluminum tray, and the moisture content was recorded directly from the instrument panel.
Frozen samples at each time point (7, 14, and 21 days) were thawed at 4±1°C for 24 h. Duck meat samples (350 g; 0, 7, 14, and 21 days) were vacuum-packed and cooked in a 70°C water bath until the core temperature reached 70°C, then immediately cooled in 18°C tap water for 30 min. Excess moisture was removed with paper towels, and cooking loss was calculated based on initial and final weights.
The samples were then cooled to room temperature and cut into six 0.5-inch-diameter strips parallel to muscle fibers for Warner-Bratzler Shear Force (WBSF) measurement using an Instron Universal Testing Machine (Model 3342) with a V-shaped blade. The machine measured the peak force (in kg) required to shear through a 1.27-cm-thick core once, perpendicular to the fibers, with the average peak shear force from six cores indicating muscle tenderness.
The VBN content in duck meat was determined using a modified Conway microdiffusion dish method. Meat samples (10 g) were homogenized with distilled water, filtered, and the filtrate was used in the diffusion dish along with H3SO3 and an indicator. After incubation at 37°C for 2 h, the absorption solution was titrated with H2SO4 to calculate VBN content.
Minced duck meat (3 g) was mixed with butylated hydroxytoluene (BHT; 60 μL) and ultrapure water (9 mL), homogenized (15 s, 14,000 rpm/min), filtered, and 1 mL filtrate was reacted with 2 mL Trichloroacetic acid/Thiobarbituric acid (TCA/TBA) mixture at 90°C for 15 min. After cooling, the mixture was centrifuged (10 min, 1,000×g/min), and malondialdehyde (MDA) content was determined by measuring absorbance at 531 nm.
According to Hoa et al. (2024), 3 g of ground duck meat was placed in a 20 mL vial with 3 mL of 20% NaCl solution and mixed. An internal standard (1.0 μL, 2-methyl-3-heptanone, 0.816 mg/mL in methanol) was added, and the vial was sealed. Flavour compounds were extracted using an SPME instrument (Supelco, Sigma-Aldrich, St. Louis, MO, USA) with a carboxy polydimethylsiloxane (75 μm) fibre at 60°C for 60 min. The volatiles were desorbed at 250°C for 5 min at a 10 mL/min split flow rate and separated on a 30 m×0.25 mm×0.25 μm capillary column using a GC-MS (Agilent 8890B GC and 5977B MSD, Agilent Technologies, Santa Clara, CA, USA). The oven temperature was set to 40°C for 5 min, then increased to 250°C at 8°C/min and held for 5 min.
The retention index (RI) was used to qualitatively analyze meat sample volatiles using 14 NIST databases and n-alkanes (C7–C40) as external references, calculating RI values per (Cui et al., 2023). The relative abundance of each volatile component was determined from peak areas in the meat sample using 2-methyl-3-heptanone as the internal standard. The content of volatile substances (μg/kg) was calculated using the formula:
where C0 is the internal standard concentration (μg/μL), m is the meat sample mass (g), Ai is the analyte peak area, and A0 is the internal standard peak area. Quantitative analysis was performed by comparing peak areas with those in the NIST14.L library.
Statistical analysis of meat quality data (including pH, color, moisture content determination, cooking loss, etc.) was performed using IBM SPSS Statistics 24 (SPSS Institute, Chicago, IL, USA) using one-way analysis of variance (One-way ANOVA) and Duncan’s multiple comparison test. Data on volatile flavor compounds were analyzed using generalized linear models in IBM SPSS version 24 (SPSS Institute). Multifactorial analysis of variance (M-MANOVA) was performed with the time point of each group as a fixed factor and volatile flavor compounds as the dependent variable. The significance level was set at p<0.05. Chiplot (https://chiplot.online/) was used to draw shear force grouped box plots. Volatile compound concentrations were calculated from GC-MS peak areas using 2-methyl-3-heptanone as the internal standard. The normalized data were uploaded to MetaboAnalyst 6.0 (https://www.metaboanalyst.ca/docs/RTutorial.xhtml) for statistical analysis. Log10 transformation and auto-scaling were applied prior to multivariate analysis, including PLS-DA, VIP, and heatmap visualization. The data points shown in Fig. 1 represent individual biological replicates. Origin 2018b 64Bit was used for VBN and TBARS graphics; flavordb2 (https://cosylab.iiitd.edu.in/flavordb2/search) was used to query the odour threshold and odour characteristics of flavour substances.
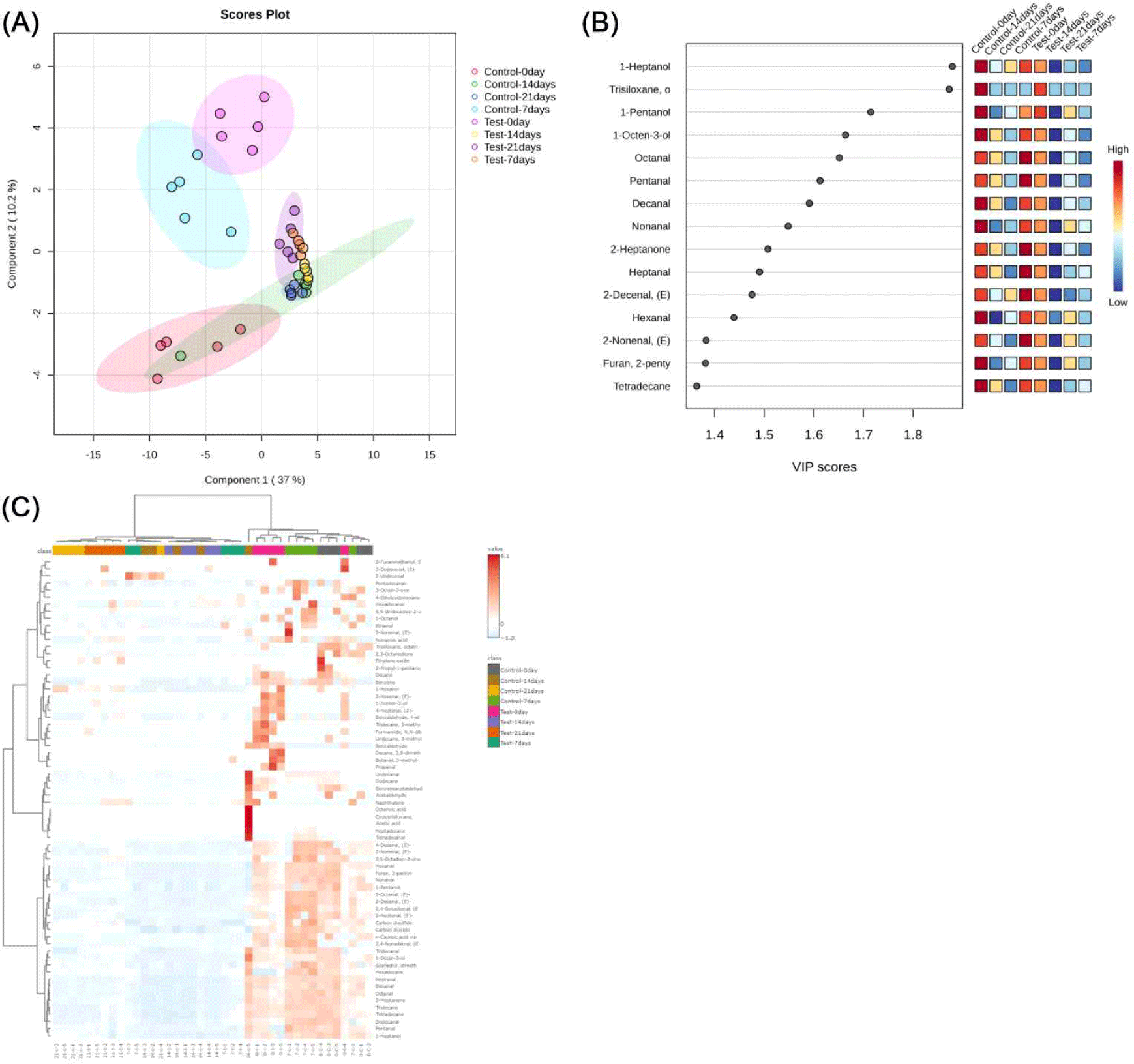
Results and Discussion
The quality data sheet of duck meat during storage (0, 7, 14, and 21 days) shows changes in pH, color (CIE L*, CIE a*, CIE b*), moisture content, cooking loss, and shear force. The pH values remained stable between 6.03 and 6.07, consistent with poultry meat pH stability (Zhou et al., 2010). Moisture content decreased slightly over time, with no significant differences between control and experimental groups, indicating minimal storage impact on moisture (Chang et al., 2024). In terms of color, the test group’s L-value (brightness) was 58.97, lower than the control group’s 63.03, indicating darkening with storage (Wereńska, 2024). The a-value (redness) increased significantly, reaching 11.47 at 21 days, higher than the control group’s 9.94, suggesting increased redness over time. The b-value (yellowness) varied slightly, with values between 19.89 and 21.88 for both groups, aligning with chromaticity changes during storage (Zhang et al., 2013). Cooking loss increased in the test group with prolonged storage, indicating a decline in meat quality.
Under vacuum cooking, the cooking loss of duck breast increased with storage time (Table 2). After 21 days of frozen storage, the cooking loss of the fish-meal group (37.02±0.77%) was significantly higher than that of the control group (35.31± 0.80%, p<0.05). Fish meal is rich in soluble muscle proteins, peptides, and collagen, which can insert between myofibrillar proteins or compete with them for water binding, thereby disrupting the original protein network and reducing the number of water-binding sites (Nuñez et al., 2021). Additionally, on day 14, the pH of the fish-meal group dropped to 5.99, approaching the isoelectric point range of muscle proteins (pH 5.4–5.8). At this pH, electrostatic repulsion among proteins is weakened and structural contraction is enhanced, further promoting water release during heating (Huff-Lonergan and Lonergan, 2005). Moisture content showed only a slight and non-significant decrease over storage time.
Control group: control feed (commercial feed); experimental group: feed supplemented with fish meal.
Different superscript letters (a–c) within the same row indicate significant differences among the means at p<0.05.
Addition of fish meal significantly accelerated lipid oxidation (Fig. 2A). The MDA value of the experimental group increased from 0.91±0.01 mg/kg on day 0 to 2.04±0.01 mg/kg on day 21, which was significantly more than that of the control group (1.83±0.02 mg/kg, p<0.05). Thus, the high content of polyunsaturated fatty acids in fish meal may lead to the production of secondary lipid oxidation products, such as malondialdehyde and other aldehydes. These compounds can cross-link with myofibrillar proteins, causing denaturation and aggregation, which in turn reduces the water-holding capacity (Estévez, 2011).
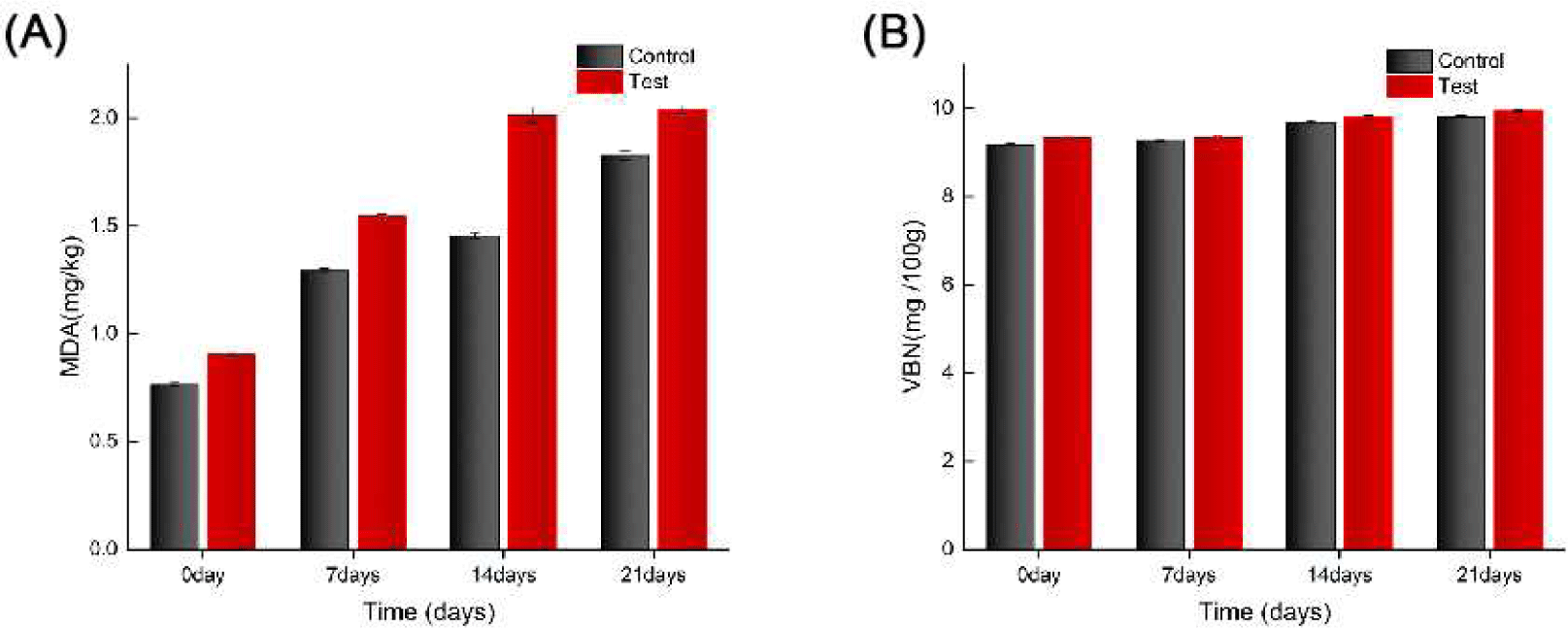
The study assessed the rapid oxidation of duck breast using the TBARS method to measure MDA levels. MDA levels increased significantly with longer storage time, indicating increased lipid oxidation, consistent with previous research (Zheng et al., 2019). Lipid oxidation, which produces MDA and other byproducts that affect meat quality, was more pronounced in the fish meal group, aligning with studies showing that raw materials rich in polyunsaturated fatty acids (PUFA), such as fish meal, accelerate lipid oxidation (Coronado et al., 2002). This study is consistent with a systematic study by Channon et al. (2007) on the effects of fishmeal diets on fatty acid composition and lipid oxidation in pork. Fishmeal significantly alters the fatty acid composition of pork by enriching it with PUFA, particularly by increasing the content of long-chain n-3 PUFAs such as eicosapentaenoic acid (EPA; C20:5) and docosahexaenoic acid (DHA; C22:6). In the study by Channon et al. (2007) it was shown that the use of PorcOmega™ (stabilized tuna fishmeal) as a fishmeal supplement increased n-3 PUFA levels in pork by approximately three times. However, this change in fatty acid composition also raises the issue of oxidative stability. Due to the multiple double bonds in the PUFA molecule, it is highly susceptible to free radical attack, which results in the formation of primary lipid oxidation products, which are further decomposed into secondary products such as MDA, leading to elevated TBARS values and quality deterioration. This oxidative process is especially significant during refrigerated or frozen storage. In addition, the literature suggests that although there are nutritional advantages to a decrease in the n-6:n-3 PUFA ratio in pork, an elevated degree of unsaturation of fatty acids significantly increases the risk of lipid oxidation (Channon et al., 2007).
The VBN value, a key indicator of protein decomposition and meat freshness, also showed a significant upward trend with longer storage time. The test group’s VBN value increased notably after 21 days, reflecting increased protein decomposition and a significant decrease in meat freshness, consistent with meat spoilage characteristics (Jiang et al., 2016). Elevated VBN values indicate protein degradation and a gradual deterioration of meat quality during long-term storage (Zhou et al., 2010).
During frozen storage from day 0 to day 21, the distribution of shear force in the control group was generally stable, but the median increased gradually, indicating a certain degree of hardening of the meat during storage. In the fishmeal-added group (test group), the distribution of shear force varied more significantly, especially between day 14 and day 21 (Fig. 3), suggesting that fishmeal may affect the stability of the muscle structure of duck meat under frozen vacuum storage conditions.
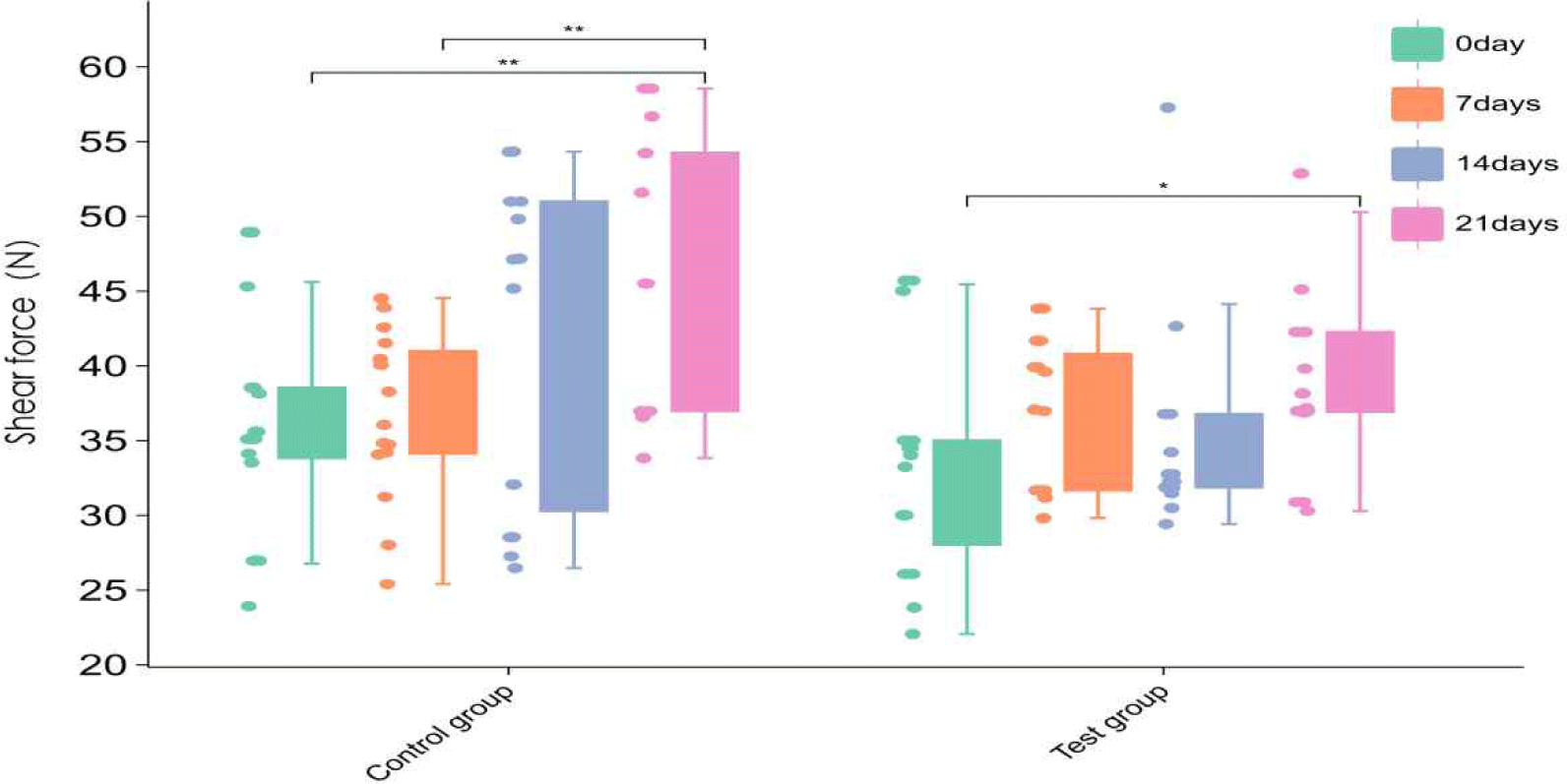
Shear force is a key indicator of meat tenderness and directly affects consumer acceptance (Tornberg, 2005). Studies have shown that when the shear force value exceeds 50 N, it is often associated with negative sensory experiences such as deterioration of texture and difficulty in chewing. In the present study, the fishmeal group had greater shear force fluctuations and higher mean values in the later stages, which may be related to its modulation of oxidative aggregation of myofibrillar proteins or inhibition of degradation of muscle structures by proteolytic enzyme systems such as calpain (Estévez et al., 2006; Pinotti et al., 2023).
In addition, fishmeal is rich in unsaturated fatty acids, the oxidation products of which may form crosslinked aggregates with proteins that are not easily degraded, thereby enhancing the structural rigidity of meat tissue (Lund et al., 2011). These protein cross-linking phenomena may lead to changes in muscle structure, such as the length of muscle segments and the integrity of muscle membrane, which need to be further verified by transmission electron microscopy or muscle proteomics (Zhang et al., 2013). Therefore, the effect of fishmeal on the shear force of duck meat during storage is not only reflected in the mechanical properties, but may also indirectly act on the flavor perception and overall sensory evaluation of consumers.
Table 3 presents volatile flavor compound profiles in duck breast meat from control and fish meal-fed ducks over 21 days of storage (n=5). Aldehydes, mainly lipid oxidation products (e.g., hexanal, nonanal), were dominant in the control group, showing a significant decline by day 21. In contrast, the fish meal group exhibited lower initial aldehyde levels, indicating reduced lipid oxidation susceptibility. Ketones such as 2,3-octanedione followed a similar trend. Esters, particularly n-caproic acid vinyl ester, were more abundant in the fish meal group, likely due to enzymatic esterification of fish-derived fatty acids, enhancing flavor richness. Alcohols (e.g., 1-hexanol, 1-octen-3-ol) and aromatic compounds were also higher in the fish meal group, contributing to flavor complexity. Furans and hydrocarbons generally decreased over time, especially by day 14. Volatile compounds peaked around day 7, then stabilized or declined by day 21. Overall, fish meal supplementation imparted a distinct volatile profile with elevated esters and reduced aldehydes, highlighting its role in modulating flavor development during storage (Lorenzo and Domínguez, 2014).
Control group: control feed (feed without fish meal), n=5; Experimental group: (feed with fish meal), n=5.
In this study, PLS-DA was used to explore the differences in flavor components of duck meat steamed in vacuum at low temperature under different storage conditions (Fig. 1A). The score plots showed that the first two principal components explained 37.0% and 10.2% of the flavor variance, respectively, which was low, but effectively revealed the trend of flavor changes with storage time and conditions. The control samples at 0, 7, 14 and 21 days of storage showed significant clustering, indicating that flavor evolved gradually during fronzen storage.
At the beginning of storage (0 and 7 days), the significant differences between the test and control groups may be related to the unsaturated fatty acids and their metabolites in fishmeal (Suárez-Medina et al., 2024). By 14 and 21 days, the samples of the test group tended to concentrate, suggesting a gradual stabilization of the effect of fishmeal on flavor, which may be related to the oxidation of fatty acids or the volatilization of specific flavor substances (Grigorakis et al., 2010). After 21 days of storage, the flavors of the two groups stabilized but remained significantly different, with the test group developing a distinctive flavor profile.
In the field of food science, fatty acid oxidation is one of the key mechanisms affecting flavor formation in meat (Mottram, 1998). In this study, the VIP score of PLS-DA was used to analyze the effects of different storage times on the flavor compounds of low-temperature vacuum-cooked duck meat (Fig. 1B). The results showed that 1-heptanol, trisiloxane (octamethyltrisiloxane), 1-pentanol, 1-octen-3-ol, octanal, pentanal, heptanal, nonanal, decanal, 2-heptanone, 2-decenal, 2-nonenal, 2-pentylfuran, hexanal, and tetradecane had higher VIP scores, which were the key compounds to distinguish between different storage time groups. Although Component 1 (explaining 37% of the variance) alone provided only partial separation of the storage time clusters, incorporating Component 2 (10.2%) resulted in clearer time-dependent grouping, especially between the 0-day and 21-day samples (Fig. 1A). Thus, while the VIP plot reflects importance in Component 1, the interpretation of both components together provides a better explanation of the dynamic changes in volatile profiles across storage periods. These substances are mostly products of thermal oxidation of fatty acids and play a decisive role in meat flavor (Liu et al., 2020; Shahidi and Hossain, 2022; Yang et al., 2018). Unsaturated fatty acids such as linoleic acid generate hydroperoxides during storage and heating, which are further degraded to produce aldehydes, ketones, alcohols, and furans, among which octanal, heptanal, and nonanal are considered to be the representative odorants of “overcooked flavor” (Li et al., 2025). The VIP score graphs showed that 1-heptanol and trisiloxanes etc. increased with increasing storage time in the test group. 1-heptanol and trisiloxane in the test group increased with storage time and were significantly higher than those in the control group, suggesting that lipid oxidation contributed to the accumulation of flavor substances. Duck meat rich in unsaturated fatty acids undergoes oxidative reactions during storage to produce a variety of key aroma compounds (Cheng et al., 2024). Overall, fatty acid oxidation plays an important role in the flavor evolution of duck meat, and the related markers can be used as an important basis for assessing flavor changes and optimizing storage conditions.
Fig. 1C demonstrates the characterization of volatile compounds in low-temperature vacuum-cooked duck meat at different storage times and feeding conditions. In the early stage (day 0), nonanal (with citrus aroma) and undecane (neutral, waxy odor) in duck meat were the flavor substances underlying the natural aroma. As storage was extended to days 7 and 14, fat oxidation and protein degradation reactions introduced flavor substances such as (Z)-2-nonenal and acetic acid, which imparted a more complex fatty, grassy, and acidic flavor to the meat. A significant rise in acids (e.g., acetic acid) and sulfide-containing compounds (carbon disulfide) at day 21 was strongly associated with spoilage and off-flavors. Flavor changes were more drastic in the fishmeal group compared to the control group, especially in the later storage phase when more compounds associated with roasted and nutty flavors (e.g., (Z)-4- heptanol, 1-penten-3-ol) appeared, suggesting that dietary fishmeal significantly influences the process of flavor formation in duck meat (Jayasena et al., 2013).
Fishmeal is rich in a variety of unsaturated fatty acids, especially PUFAs such as linoleic acid (C18:2n-6), linolenic acid (C18:3n-3) and EPA (C20:5n-3). These fatty acids are highly susceptible to spontaneous or enzymatic oxidation during storage, generating lipid peroxides, which are further cleaved to form volatile aldehydes and ketones and other key flavor substances. Initial oxidation of linoleic acid forms intermediates such as 13-hydroxyoctenoic acid (13-HPODE) and 9-HPODE, which are subsequently cleaved to form aldehydes with grassy and fatty flavors, such as hexanal, nonanal, nonenal, and pentanal, etc. Oxidation of EPA generates (E,E)-2,4-decadienal, heptanal, etc., which have strong sensory activities. In addition, fatty acids can further form alcohols, ketones and acids through β-oxidation or interaction with myofibrillar proteins (Sohaib et al., 2017).
When investigating the mechanism of fatty acid oxidation during the fermentation of traditional fish sauce, Wang et al. (2018) found that hexanal in the oxidation products of linoleic acid was highly negatively correlated with the change in its content (R=–0.9587), suggesting that fatty acids are the direct precursors of these flavor substances. This finding supports the fishmeal-induced flavor changes in duck meat from the lipid degradation pathway in fermented foods (Wang et al., 2018).
Similarly, a study by academician Beiwei Zhu’s team further revealed the effect of the interaction between fatty acids of different saturations and myofibrillar fibrillar proteins on the generation of flavor substances. The study constructed a thermal oxidation model of typical fatty acids such as oleic acid, linoleic acid and stearic acid with fish myogenin, and found that the proportion of acid compounds generated when linoleic acid was involved in the reaction was significantly higher, especially hexanoic acid was the most prominent; whereas aldehydes and alcohols were mainly generated by oleic acid, indicating that fatty acid saturation directly affects the type of its oxidation products, in which myogenin plays a role as a catalyst or a transformer (Zhao et al., 2025).
In this study, the types and abundance of volatile compounds increased significantly in the fish meal group during the late storage period. Heat map analysis clearly showed the trend of the relative contents of fat oxidation products (e.g., (Z)-2- nonenal, heptanone, acetic acid, etc.) in each treatment group during storage, with darker colors indicating higher abundance. Significant band differences were formed between different storage times and feed compositions, further confirming that the incorporation of unsaturated fatty acids in fish meal activates the lipid oxidation chain reaction, which promotes the production of characteristic flavor substances and, to some extent, influences the final quality of duck meat (Mancinelli et al., 2021).
In summary, dietary addition of fishmeal provides substrates for lipid oxidation reactions by providing abundant PUFAs, which in turn promotes the production of specific volatile aldehydes, ketones and acidic flavor substances during storage. The flavor trends and oxidation mechanisms were consistent with the results of previous studies on fish products, lipid models and fermented foods, providing a theoretical basis for further optimization of waterfowl feed formulations and enhancement of meat organoleptic quality.
Conclusion
During 21 days of vacuum storage, duck breast meat exhibited significant changes in both quality attributes and volatile flavor compounds. Physicochemical analysis showed stable pH initially, followed by a decline to near the isoelectric point by day 14, contributing to reduced water-holding capacity. Moisture content decreased slightly, while cooking loss and shear force increased, particularly in the fishmeal-supplemented group, indicating compromised tenderness and structural integrity. Color parameters revealed darkening (lower CIE L*) and increased redness (higher CIE a*) over time. Lipid oxidation, measured by TBARS (MDA), and protein degradation, indicated by VBN, increased significantly, especially in the fishmeal group, due to the high content of PUFAs. Flavor analysis demonstrated that fishmeal supplementation altered the volatile profile, with increased esters, alcohols, and acids, and decreased aldehydes. Key flavor compounds such as hexanal, nonanal, and 1-octen-3-ol were identified as markers of lipid oxidation. Multivariate analysis (PLS-DA and VIP) confirmed that storage time and dietary fishmeal significantly influenced flavor development. Overall, while fishmeal enhanced flavor complexity, it also accelerated oxidative spoilage and quality deterioration, highlighting the need for antioxidant strategies to balance nutritional benefits and meat quality during storage.
