Introduction
Recently, consumer interest in quality poultry products has increased significantly in global markets, including Korea, where chickens remain one of the most widely consumed animal proteins (FAO, 2024). In poultry muscle cuts, the muscle fiber type, metabolic activity, and biochemical composition of the breast and leg muscles vary, resulting in differences in amino acid composition and nutritional value (Chen et al., 2015; Cheng et al., 2022; Straková et al., 2006). Understanding the composition and functional properties of these muscles is essential for quality control, product development, and the development of alternative protein sources.
The cultured meat industry is emerging as a promising solution for addressing issues of food security, environmental sustainability, and animal farming ethics (Kumar et al., 2021; Treich, 2021). One of the core aspects of cultured meat production is the isolation and expansion of skeletal muscle satellite cells (SCs), which can differentiate into myotubes under appropriate conditions (Ben-Arye and Levenberg, 2019; Syverud et al., 2014).
Cultured meat technology has emerged as a sustainable alternative to traditional livestock farming, relying on the expansion and differentiation of SCs, which ultimately differentiate into myotubes and muscle tissues (Post et al., 2020). However, the success of in vitro myogenesis largely depends on the purity of the SCs isolated during primary culture (Joo et al., 2022; Yin et al., 2013). Many non-muscle stem cells, particularly fibroblasts, hinder myotube development by competing for nutrients and space (Asakura et al., 2002). Recent studies have shown that higher SC purity promotes differentiation and myotube fusion; however, little attention has been paid to how this purity affects the biochemical or nutritional characteristics of the final tissue, particularly the amino acid composition (Kim et al., 2022; Stout et al., 2022). In addition to the morphology, the biochemical composition of cultured tissues is critical for assessing their equivalence to conventional meat (Fraeye et al., 2020). Amino acids influence not only nutritional value but also sensory characteristics such as sweetness, bitterness, and umami (Kawai et al., 2002). Therefore, understanding how SC purity affects the amino acid composition is crucial for improving the quality of cultured meat.
Therefore, the objective of this study was to compare the amino acid composition of broiler chicken breast and leg meat, as well as in vitro cultured myotubes derived from SCs of different purities (60%–69%, 70%–79%, 80%–89% and 90%–100%). Because SC purity influences not only myotube differentiation but also cellular metabolism, we further aimed to clarify how purity-dependent differences contribute to shifts in amino acid composition. By addressing this link, our study provides fundamental insights into optimizing the nutritional fidelity of cultured poultry meat and enhancing its potential as a viable meat substitute.
Materials and Methods
Breast (M. pectoralis major) and whole leg muscles were obtained from 4-wk-old Ross broiler chickens (n=3) immediately after slaughter at a commercial slaughterhouse, in accordance with the act on the sanitary control of livestock products, Korea. SCs for cultured tissues were collected from 13-d-old fertilized chicken embryos (n=3). Muscle tissues were dissected under sterile conditions, and the bones, cartilage, and connective tissues were carefully removed. Each sample was surface-sterilized by quick washing with 70% ethanol and then immersed in Hanks’ balanced salt solution (HBSS; GibcoTM, Grand Island, NY, USA) supplemented with 1% penicillin–streptomycin (P/S; Welgene, Gyeongsan, Korea).
The enzyme digestion protocol described by Kim et al. (2022) was slightly modified for isolation of chicken SCs. Briefly, muscle tissue was minced with surgical scissors in a sterile culture dish until it reached a fluid state. Approximately 1 g of minced tissue was transferred to a pre-weighed 50 mL conical tube. A solution of 0.2% type II collagenase (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with Dulbecco’s modified Eagle medium (DMEM; Welgene) at a ratio of 1:10 (w/v) was added and the minced tissue was incubated at 37°C in a water bath for 1 h. During incubation, the pipette suspension was collected every 15 min using a 10 mL syringe to enhance dissociation. The mixture was centrifuged at 300×g for 5 min at 4°C. The supernatant was discarded and the remained pellet was resuspended in 5 mL of 0.25% trypsin-EDTA (Welgene) followed by incubation at 37°C for 20 min with gently shaking every 5 min. One milliliter of fetal bovine serum (FBS; Corning, Glendale, AZ, USA) was added along the tube wall to neutralize trypsin activity, and the mixture was resuspended in phosphate-buffered saline (PBS; containing 1% P/S) to adjust to a final volume of 35 mL. The mixture was then filtered through 70 μm and 40 μm cell strainers (SPL life science, Pocheon, Korea), respectively, to remove tissue debris. After centrifuging the filtrate at 300×g for 5 min at 4°C, the pellet was washed with 1 mL ACK lysis buffer (GibcoTM, Grand Island, NY, USA) to remove red blood cells, followed by incubation on ice for 10 min. To terminate the reaction, 20 mL of PBS was added and the mixture was centrifuged under the same conditions. After removing the supernatant, the pellets were resuspended in the growth medium (GM). The cell viability was assessed using 0.4% trypan blue (GibcoTM) staining. The GM was prepared with 20% FBS, 5 μM p38 inhibitor (MedChemExpress, Monmouth Junction, NJ, USA), 1% P/S, 5 ng/mL basic fibroblast growth factor (BioLegend, San Diego, CA, USA) and 1 mL Normocin (InvitrogenTM, San Diego, CA, USA) in DMEM.
The pre-plating method described by Kim et al. (2022) was slightly modified to account for differences in the adhesion times of fibroblasts and SCs. Freshly isolated cell suspensions were seeded into T75 culture flasks at a density of 5×106 cells/cm2 and incubated at 41°C for 2 h. The unattached cells (rich in SCs) were collected, transferred to another T75 culture flask, and further incubated for 1 h. During incubation, the culture flasks were gently shaken for 20 s every 8 min at 60 rpm using a SciLabShake shaker. The supernatant containing purified stem cells was collected after centrifuging at 300×g for 5 min at 4°C. The attached cells were collected using trypsin treatment, and trypsin activity was neutralized by adding FBS. The pellets were collected after the centrifugation at 300×g for 5 min at 4°C. The SCs in both the pellets collected from the supernatant and the cells attached to the culture flask were counted after immunohistochemistry using antibodies against Pax7 (DSHB, Iowa City, IA, USA) and anti-IgG conjugated with AlexaFluor 594 (Thermo Fisher Scientific). All cells were visualized using Hoechst staining (50 μg/mL) for 30 min at room temperature. Different SC purity groups were prepared by combining the pellets collected from the supernatant and the attached cells in the pre-plating flask. The different purity groups were 60%–69%, 70%–79%, 80%–89%, and 90%–100% for breast SCs and 60%–69%, 70%–79%, and 80%–89% for leg SCs. The culture flasks containing cells were incubated in a 5% CO2 incubator at 41°C with seeding density of 5×103 cells/cm2. The medium was replaced every 48 h, and all flasks were pre-coated with type I collagen to support cell attachment and differentiation. SCs were cultured in GM until they reached 80%–90% confluence. The GM was replaced with differentiation medium (DM; 2% FBS, 1% P/S, and 1 mL Normocin in DMEM) to induce differentiation. Cells were cultured at 41°C for 5–7 d, and the medium was replaced every 2 d.
Differentiated myotubes were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and immunostained with myosin heavy chain to assess myotube morphogenesis (MF20, DSHB). To visualize myotubes, anti-IgG2b conjugated with AlexaFluor 488 (Thermo Fisher Scientific) was used as the secondary antibody. Nuclei were stained with Hoechst, as described above. The cultured tissue was visualized using a confocal scanning laser microscope (TCS SP8 STED, Leica Biosystems, Wetzlar, Germany). At least five random microscopic fields per well were analyzed, resulting in a total of 15–20 fields per experimental group across all biological replicates. Myotube morphology, such as myotube length (μm), and myotube thickness (μm), was evaluated using Image Pro Plus (Media Cybernetics, Rockville, MD, USA). Nuclei were counted to evaluate the fusion index (the percentage of nuclei located within multinucleated myotubes relative to the total number of nuclei).
Amino acid composition analysis was performed according to the method described by Park et al. (2025), with minor modifications. In detail, 0.2 g of dried chicken muscle tissue samples (both breast and leg) as well as differentiated myotube tissues from each SC purity group were accurately weighed. The samples were hydrolyzed by adding 5 mL 6 N hydrochloric acid and incubating at 110°C for 24 h. The hydrolysates were then filtered through a Whatman No. 1 filter paper, and the filtrate was adjusted to a final volume of 10 mL using distilled water. After thorough vortex mixing, 1 mL of the prepared solution was passed through a 0.2-μm membrane filter (Phenomenex, Torrance, CA, USA) to remove any remaining particulate matter. Amino acid concentrations were quantified using an automatic amino acid analyzer (Dionex Ultimate 3000, Thermo Fisher Scientific). The chromatographic separation was achieved on an Inno C18 column (150×4.6 mm, 5.0 μm; Youngjinbiochrom, Sungnam, Korea). Gradient elution was performed using two solvents: solvent A, 40 mM sodium phosphate buffer (pH 7); and solvent B, a mixture of water, acetonitrile, and methanol (10:45:45, v/v/v). The gradient program was as follows: 95% solvent A for 0 min, 45% solvent A for 24 min, 20% solvent A for 25 min, and 95% solvent A for 34.5 min. The flow rate was maintained at 1.5 mL/min, and the column temperature was set at 40°C. Amino acids were detected using a fluorescence detector employing two derivatizing agents: o-phthaldialdehyde (OPA; Agilent Technologies, Santa Clara, CA, USA) and 9-fluorenylmethoxycarbonyl chloride (FMOC; Agilent Technologies). OPA-derivatized amino acids were detected at excitation/emission wavelengths of 340/450 nm, whereas FMOC-derivatized amino acids were detected at 266/305 nm, respectively. Quantification was performed using five-point calibration curves constructed with an Amino Acid Standard (WAT088122, Waters, Milford, MA, USA). The concentrations of both essential and nonessential amino acids were determined based on calibration standards. Amino acid data are expressed as relative percentages of the total amino acids.
All experiments were repeated at least thrice. Data are presented as mean±SE. Statistical analysis was performed using one-way analysis of variance, followed by Tukey’s post hoc test, using SAS (v. 9.4, SAS Institute, Cary, NC, USA). p<0.05 was considered statistically significant.
Results and Discussion
An enzyme digestion method and a two-step pre-plating protocol were successfully used to isolate SCs from chicken breast and leg muscles. Immunofluorescence staining with Pax7 and Hoechst clearly identified the SCs and total cell nuclei (Fig. 1). Depending on the proportion of SCs to total cell nuclei, cells isolated from chicken breast meat were classified into four purity grades: 60%–69%, 70%–79%, 80%–89%, and 90%–100%. Cells isolated from chicken leg meat were classified into three purity grades: 60%–69%, 70%–79%, and 80%–89%. Fig. 1 shows representative immunofluorescence images of SCs cultured from chicken breast and leg muscles at different purity levels. Under the same isolation conditions, the proportion of SCs in cultures derived from chicken breast was slightly higher than that in cultures derived from leg muscles.
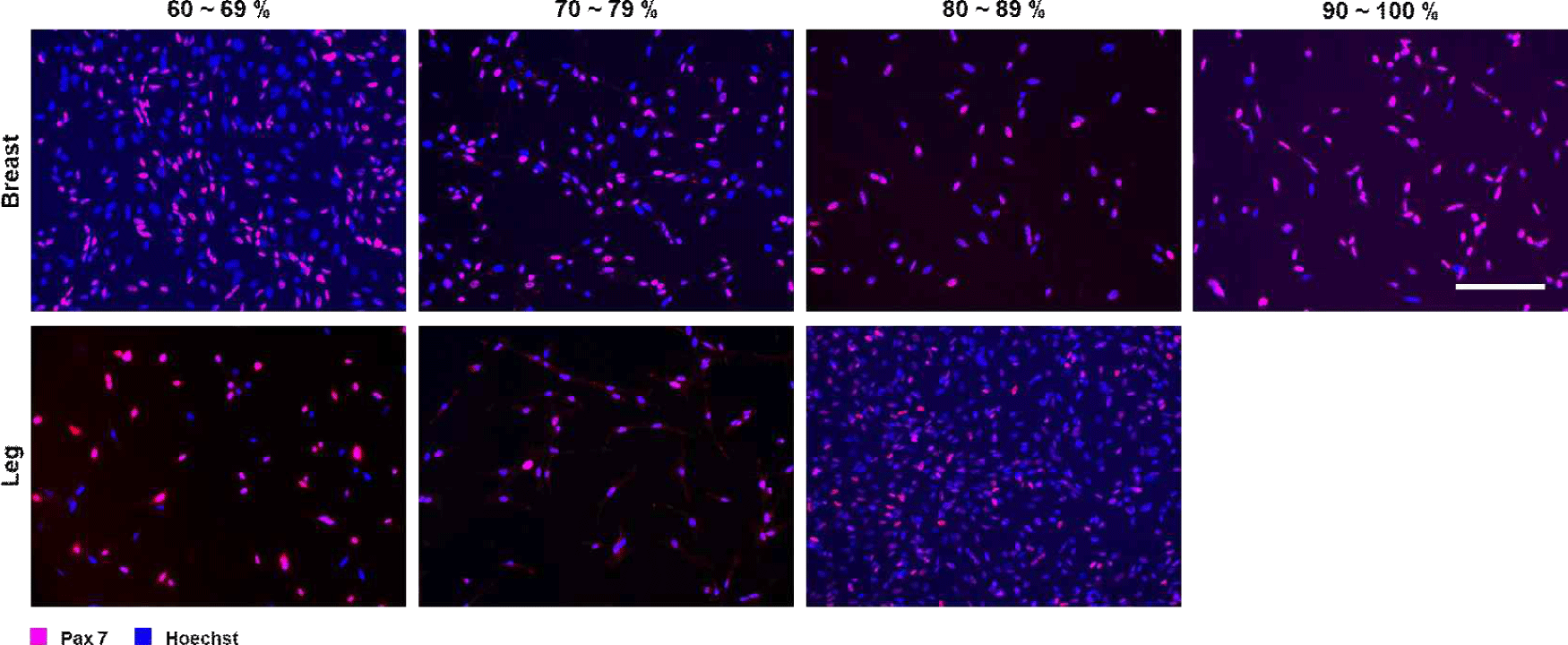
The purity of SCs is critical for subsequent muscle-forming properties, while the microenvironment constructed by the proliferative secretions of other non-SCs is also essential for the muscle regeneration process (Bentzinger et al., 2012; Yin et al., 2013). Inhibition of PDGFRα+ signaling has been shown to reduce the number of fibroblasts, which in turn promotes SC proliferation and contributes to skeletal muscle remodeling (Thooyamani and Mukhopadhyay, 2021). Although some subpopulations of fibroblast/adipogenic progenitor cells increase the rate of differentiation of muscle-forming progenitor cells in response to injury (Joe et al., 2010), the proliferation of other non-SCs generates non-muscle-like cells, such as ectopic adipocytes, by competing for nutrients and space, with considerable effects on muscle homeostasis (Uezumi et al., 2010). A similar purity-dependent trend has been observed in bovine and porcine SC isolates, in which various methods are used before proliferation culture to increase the purity of SCs and reduce contamination by non-myoblasts, resulting in more homogeneous myotube differentiation (Ding et al., 2017; Skrivergaard et al., 2021; Stout et al., 2022).
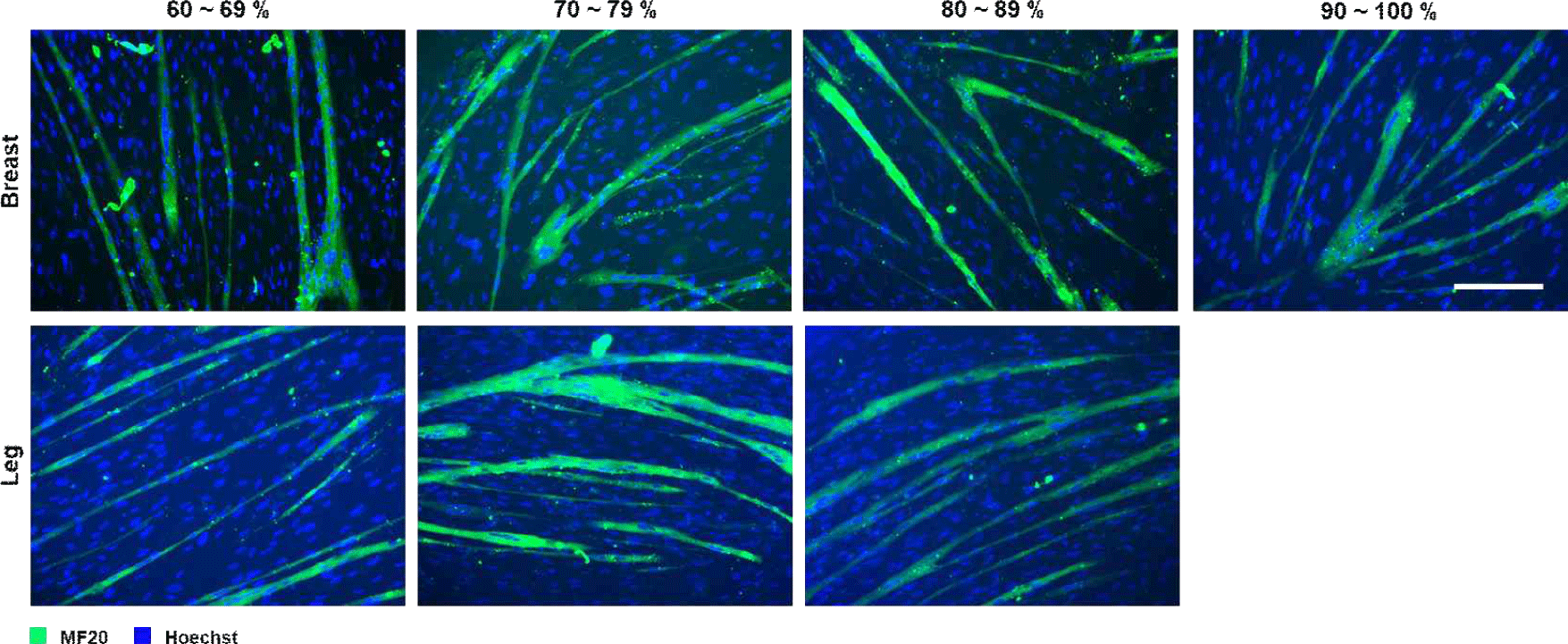
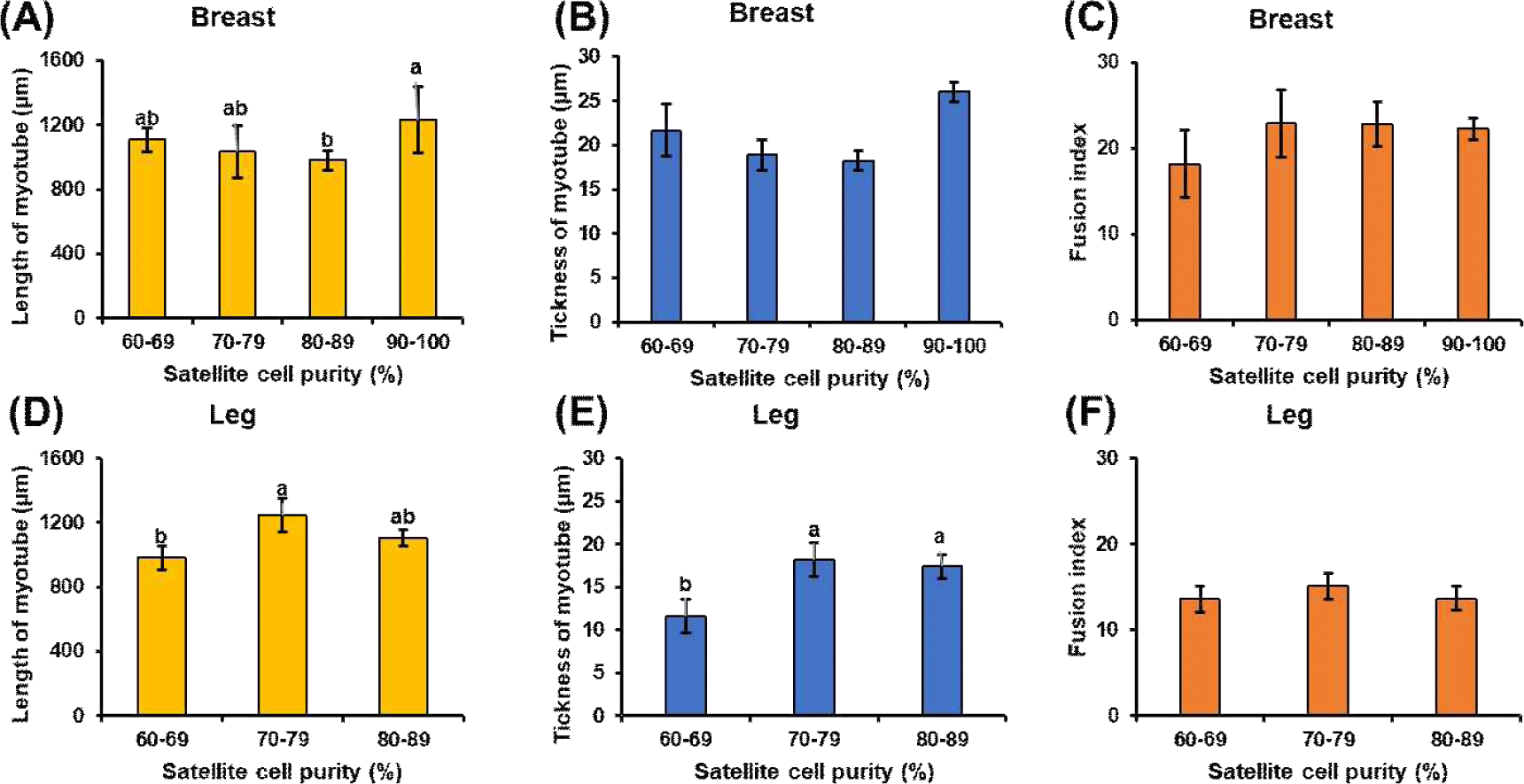
Following induced differentiation, SCs from all the purity groups successfully formed myotubes. Representative immunofluorescence images of myotubes from SCs cultured from chicken breast and leg muscles at different purity levels are shown in Fig. 2. However, the degree of differentiation and morphological integrity varied with SC purity. In cultured tissues derived from breast and leg muscles, myotubes from the high-purity group (≥80%) were longer, more uniformly arranged, and multinucleated, whereas those from the low-purity group (60%–69%) were thinner and irregular. Quantitative morphological analysis revealed that myotube characteristics improved significantly with increasing SC purity (Fig. 3). In breast muscle–derived myotubes, the 90%–100% purity group showed significantly greater length compared with the 80%–89% group (p<0.05). However, no significant differences were observed between the 90%–100% group and the lower-purity groups (60%–69% and 70%–79%), which were statistically intermediate (p>0.05). No significant differences in myotube thickness or fusion index were observed among the purity groups (p>0.05). Correspondingly, in cultured tissues derived from leg muscles, myotube thickness increased with increasing purity levels (p<0.05), with myotube length reaching its maximum at 70%–79% purity (exceeding 1,200 μm) and significantly higher than in the low-purity group (p<0.05). The fusion index increased from the lowest to the highest purity group for both muscle types, although no significant differences were observed. These results are consistent with those of previous studies showing that SC purity significantly affects myotube composition, as contaminating fibroblasts secrete extracellular matrix components that disrupt myotube alignment (Bentzinger et al., 2012; Ding et al., 2017; Stout et al., 2022). In addition, the morphological consistency of breast muscle–derived SCs is slightly higher than that of leg muscle–derived SCs, which may reflect intrinsic differences in the composition of myofiber types; breast muscle is predominantly composed of glycolytic type IIb fibers (Kim et al., 2008; Smith and Fletcher, 1988; Verdiglione and Cassandro, 2013), whereas leg muscles contain more oxidized type I and IIA fibers (Cheng et al., 2022). This difference in fiber type affects SC proliferation and differentiation, which may explain the difference in the optimal purity required for maximum myotube lengthening.
Regarding the amino acid composition within the cultured tissues, glutamic acid was the most abundant amino acid in both breast and leg muscle–derived cultured tissues, followed by arginine and leucine, whereas proline had the lowest proportion. Glutamate supports cellular metabolism and protein synthesis and is the most abundant amino acid in both conventional muscle tissues and cultured chicken tissues, which is consistent with the findings of Joo et al. (2022). Importantly, hydroxyproline—a characteristic component of collagen—was not detected in the cultured tissue samples, indicating a notable depletion of collagenous proteins. Since connective tissues are particularly rich in collagen, which is composed predominantly of proline, glycine, and hydroxyproline within its triple-helix structure, the removal of connective tissue during satellite cell isolation and subsequent culture provides a plausible explanation for the markedly reduced proline fraction compared with natural muscle tissue (Shoulders and Raines, 2009). In cultured tissues derived from leg muscle, methionine content was significantly higher in high-purity samples (≥70%) compared to low-purity samples (60%–69%; p<0.05). Glutamic acid content was the highest in leg muscle SCs, with a purity of 70%–79%, which was significantly higher than that in the group with a purity of 60%–69% (p<0.05). The elevated glutamate content in the high-purity samples may reflect its role as a nitrogen donor and enhanced amino acid metabolism that supports protein synthesis during myotube formation, which is critical for cell proliferation and differentiation during in vitro culture (Walker and van der Donk, 2016). Other amino acids remained relatively stable across groups, with no significant differences (p>0.05).
Compared to muscle tissue, cultured tissues from both breast and leg muscles exhibited distinct compositional characteristics (Figs. 4 and 5). Regardless of muscle type, compared to chicken meat, cultured tissues had higher levels of aspartic acid, glutamic acid, threonine, valine, phenylalanine, isoleucine, and leucine (p<0.05), whereas histidine, glycine, and arginine levels were lower (p<0.05). These consistent changes indicated that in vitro muscle formation (partially regulated by SC purity) modulates the amino acid profile in a manner distinct from natural tissue development. Fortunately, the content of threonine, lysine, and proline in the cultured breast muscle tissues was similar to that in natural chicken breast muscle, whereas the threonine content in leg muscle showed no significant difference between natural muscle and cultured tissues (p>0.05). Importantly, glutamate and aspartate are major factors affecting freshness (Fuke and Konosu, 1991; Kawai et al., 2002), and their elevation in cultured tissues may enhance freshness. However, concomitant reductions in glycine and alanine—amino acids associated with sweetness perception (Kato et al., 1989; Kawai et al., 2002)—as well as lower levels of histidine and arginine, which are associated with savory and bitter tastes, suggest that the sensory properties of cultured muscle may be comparable to those of natural meat flesh. To address these imbalances, strategies such as culture medium optimization, amino acid supplementation, and metabolic engineering, may be required to combine the nutritional properties of cultured tissues with those of conventional meat (Joo et al., 2022).
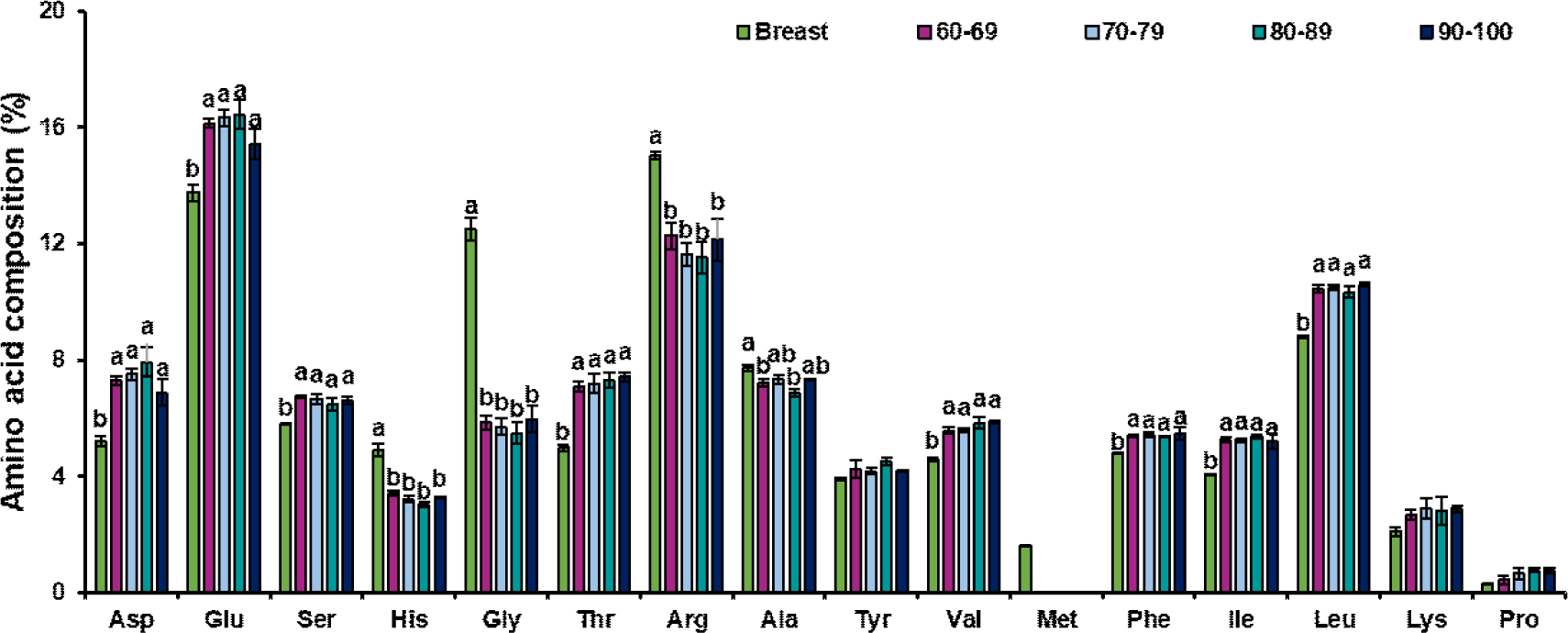
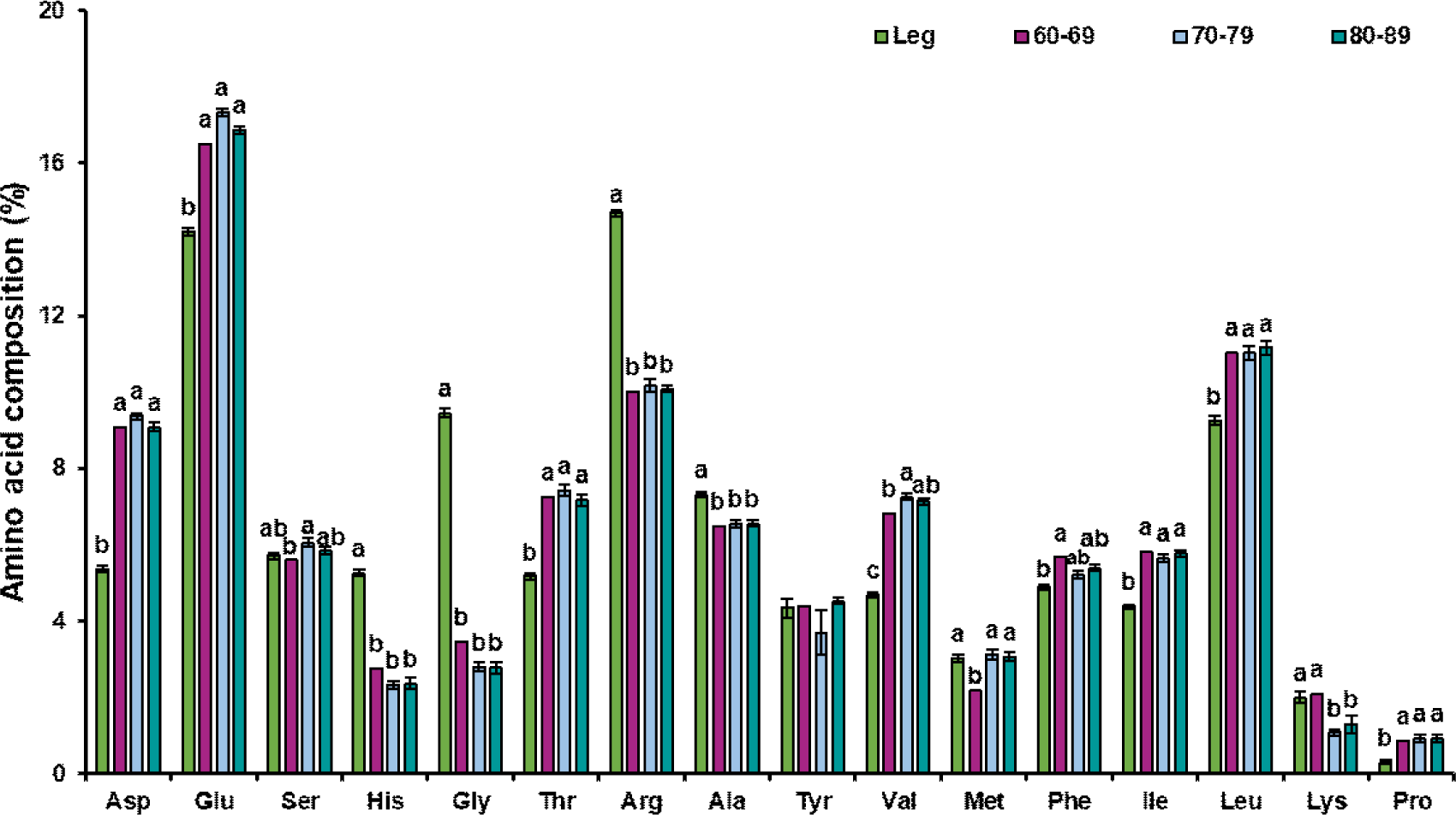
The unique amino acid profile of the cultured tissues highlights the influence of culture meat purity and culture conditions on biochemical composition. The higher essential amino acid content suggests potential nutritional value; however, the reduced content of nonessential amino acids, such as glycine, may need to be optimized to better mimic conventional meat (Joo et al., 2022). Future research should explore how the medium and culture conditions can be adjusted to balance amino acid composition while maintaining high cell culture purity to ensure a product that closely resembles natural muscle tissue in composition and function.
Conclusions
In this study, we evaluated the effects of SC purity on myotube differentiation and amino acid composition in cultured tissues and compared their amino acid compositions with those of chicken breast and leg muscles. The results demonstrated that higher SC purity significantly enhanced the morphological development of myotubes, which were characterized by increased length, alignment, and multinucleation, particularly in leg muscle–derived cultures. However, despite improved structural maturation, the amino acid profiles of the cultured tissues remained distinct from those of chicken muscle. Particularly, cultured tissues exhibited consistently elevated levels of aspartic acid, glutamic acid, threonine, valine, phenylalanine, isoleucine, and leucine and decreased levels of histidine, glycine, arginine, and alanine, regardless of SC purity or muscle type. These findings suggest that although SC purity is a critical factor in promoting muscle fiber formation and structural organization, it does not fully recapitulate the metabolic composition of muscle tissue. Further refinement of the culture conditions, including biochemical cues and extracellular matrix components, may be necessary to better mimic the nutritional and compositional characteristics of real meat.
