Introduction
Beef is highly valued for its distinctive flavor, tenderness, and juiciness, which are critical attributes influencing consumer purchasing decisions (Lee and Joo, 2022; Liu et al., 2022). Among these attributes, flavor is often cited as the most decisive criterion. Cooking methods significantly impact the flavor of meat, which in turn shape consumer preferences (Gómez et al., 2020; Xu and Yin, 2024). The flavor profile of cooked meat is primarily determined by thermal reactions, notably the Maillard reaction and lipid degradation, which generate a variety of volatile compounds contributing to its complex aroma (Sohail et al., 2022; van Ba et al., 2012).
Cooking temperature plays a pivotal role in modulating Maillard reaction products, as demonstrated by Bi et al. (2021). These reactions are temperature dependent and can produce significantly different flavor profiles at different endpoint temperatures (Roldán et al., 2015; Schwartz et al., 2022). Gagaoua et al. (2016) investigated the flavor of beef cooked at different end-point temperatures, concluding that higher cooking temperatures improved its flavor. Hanwoo, a premium Korean cattle breed, is prized by consumers for its unique marbling and distinct flavor (Hoa et al., 2023). Although volatile compounds in different cuts of Hanwoo have been well studied, limited research has addressed how doneness affects the volatile flavor profiles of specific muscles.
This study investigated the impact of cooking doneness on the volatile flavor profile of Hanwoo gluteal muscle (GM) using solid-phase microextraction gas chromatography-mass spectrometry (SPME-GC-MS). Principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) were utilized to assess volatile composition and sample distribution. Variable importance in projection (VIP) scores were used to identify key volatile markers associated with cooking doneness.
Materials and Methods
Hanwoo GM samples were obtained from Jeonju, Jeollabuk-do, South Korea. After meticulous cleaning and removal of external fat, the muscle was sectioned into uniform 3‐cm-thick pieces (n=10 per treatment). The samples were later cooked to target temperatures—rare (R; 60°C), medium (M; 71°C), and very well-done (VWD; 82°C)—using a precisely controlled water bath (DS - 21L, Dasol Scientific, Hwaseong, Korea), then promptly cooled in ice water to room temperature. A subset of each sample was immediately used for aroma analysis, while the remaining samples were stored at –20°C for subsequent processing.
Aroma volatiles were analyzed following the method described by Hoa et al. (2023). Solid-phase microextraction (SPME) was employed to extract volatile compounds from the headspace of cooked meat samples. For SPME analysis, 2.0 g portions of cooked meat were placed into 20-mL headspace vials, sealed with Polytetrafluoroethylene (PTFE)-faced silicone septa, and spiked with 1 μL of 2-methyl-3-heptanone (Sigma- Aldrich, St. Louis, MO, USA) as an internal standard. Extraction was performed using an SPME auto-sampler (PAL RSI 85, Agilent, Santa Clara, CA, USA) connected to a gas chromatography-mass spectrometry (GC-MS) system (8890 GC with 5977B MSD, Agilent). After extraction, the fiber was desorbed at 250°C for 5 min. Compounds were separated on an HP-5MS UI capillary column (30°Cm×0.25°Cmm i.d.×0.25°Cμm, Agilent) using helium as the carrier gas. The oven temperature was initially held at 40°C for 5 min, then increased at 8°C/min to 250°C, and held for 5 min. The capillary direct interface temperature was set to 250°C, with a scanning mass range of 30–500 amu at a rate of 5.27 scans/s. Volatile compounds were identified by comparing mass spectra to the National Institute of Standards and Technology (NIST) registry library (Agilent) and retention times to external standards analyzed under identical GC-MS conditions.
Statistical analyses were conducted using SPSS 24.0 (SPSS, Chicago, IL, USA). One-way analysis of variance (ANOVA) assessed overall differences among groups, followed by Duncan’s multiple range test (DMRT) to determine significant differences at p<0.05. Data are presented as mean±SD.
Multivariate analysis was performed using SIMCA 14.1 (Umetrics, Goettingen, Germany) and MetaboAnalyst 6.0 (www.metaboanalyst.ca).
Principal component analysis (PCA) is a dimensionality reduction technique used to identify patterns and relationships within complex datasets. In this study, PCA was applied to assess the distribution of meat samples across different cooking doneness levels and to detect potential outliers. Volatile compounds were quantified using internal standards and analyzed through PCA. The processed data matrix was imported into SIMCA 14.1. During data preprocessing, variables lacking significant contributions to sample pattern characterization were automatically excluded. Outliers were identified using Hotelling’s T2 statistic, where samples exceeding the T2 threshold at the 95% confidence level were classified as outliers.
A multivariate discriminant model was developed using PLS-DA after outlier removal to evaluate differences in the volatile profiles of beef samples at varying degrees of doneness. Volatile markers associated with cooking doneness were identified by calculating VIP scores and examining the spatial distribution in the biplot. Model performance was assessed using R2X (variance explained in the predictor matrix) and R2Y (variance explained in the response matrix), reflecting the model’s explanatory power for X and Y variables, respectively. To mitigate overfitting, a permutation test was performed to evaluate model robustness. The model was deemed robust if the Q2 value at the intersection of the regression line and the origin in the permutation test exceeded that of the original model. After multidimensional validation, potential volatile markers with VIP values >1 were selected.
Results and Discussion
Flavor is a crucial organoleptic attribute of beef quality, predominantly developed through chemical reactions during cooking (Fu et al., 2022). Heating induces fat oxidation and the Maillard reaction between amino acids and reducing sugars, which synergistically generate a diverse range of volatile flavor compounds (Khalid et al., 2023; Sohail et al., 2022). These include lipid oxidation derivatives, Maillard reaction products, and secondary compounds formed through their interactions, collectively contributing to the distinctive aroma and flavor profile of cooked meat (Resconi et al., 2013; Shahidi and Hossain, 2022). GC-MS is a critical tool in flavor characterization studies (Le Quéré and Lucchi, 2022).
Table 1 presents the volatile compound concentrations (μg/g) in Hanwoo GM samples at different cooking doneness levels. A total of 31 compounds, including 5 alcohols, 13 aldehydes, 8 hydrocarbons, 2 ketones, 1 furans, and 2 sulfur-containing compounds, were detected and identified in beef samples at three cooking doneness levels. The Venn diagram in Fig. 1 shows that 17 aroma compounds are common to all three cooking doneness levels, while (E)-Hexadec-2-enal, Pentadecanal, (E)-2-Octene, 3-ethyl-2-methyl-1,3-hexadiene, Tetradecanal, and Tridecanal are found only in VWD.

During cooking, alcohols—products of the thermal oxidation of fatty acid derivatives—play a crucial role in the formation of cooked meat flavor due to their low odor detection thresholds (Domínguez et al., 2019; Park and Choi, 2025). Among these, 1-Octen-3-ol levels were significantly higher (p<0.05) in the VWD group compared to other cooking doneness levels. Similarly, most aldehydes, except for Strecker aldehydes, are primarily formed through the thermal oxidation of fatty acids during cooking and contribute significantly to cooked meat aroma due to their low odor detection thresholds (Bleicher et al., 2022; Wojtasik-Kalinowska et al., 2024). In this study, eight aldehydes, including (E)-2-Heptenal, (E)-2-Octenal, Benzaldehyde, Hexanal, and Pentanal, exhibited significantly higher levels (p<0.05) in the VWD samples compared to the other doneness levels. Hydrocarbons, produced through the Maillard reaction and fatty acid oxidation, contribute less to the overall flavor of cooked meat due to their higher odor detection thresholds, which diminishes their sensory impact compared to other volatile compounds (Fu et al., 2022; Wang et al., 2023). The results revealed that the M group had significantly higher (p<0.05) levels of D-Limonene compared to the other doneness groups. Ketones, which are formed during fatty acid oxidation, also contribute less to cooked meat flavor due to their high odor detection thresholds (Dinh et al., 2021; Mottram, 1998). Notably, the VWD group exhibited significantly higher (p<0.05) levels of 2,3-Octanedione and 2-Heptanone. Furans, produced through the Maillard reaction of free amino acids with sugars or by unsaturated fatty acid oxidation, have a high odor detection threshold, reducing their contribution to the flavor profile of cooked meat (Kosowska et al., 2017; Sun et al., 2022). The VWD group showed significantly higher (p<0.05) levels of 2-pentyl-Furan than other groups. Sulfur-containing compounds, formed during the Maillard reaction, are key contributors to the distinctive flavor of cooked meat (Mottram, 1991; Park and Choi, 2025). Dimethyl sulfide levels were significantly higher (p<0.05) in the VWD group compared to the other groups.
Hanwoo is recognized for its high fat deposition capacity, and intramuscular fat (IMF) levels in beef positively influence volatile flavor compounds (Hoa et al., 2023; Hoa et al., 2024). Fat oxidation during cooking primarily drives the formation of alcohol and aldehyde flavor compounds (Dinh et al., 2021; Shahidi and Hossain, 2022). Studies have shown that the degree of doneness significantly influences the volatile flavor profile of beef (Gardner and Legako, 2018; Mallick et al., 2021), consistent with the findings of this study. Taken together, these findings suggest that cooking doneness significantly influences the type and concentration of volatile compounds produced in Hanwoo GM samples, with distinct differences in flavor compound profiles across doneness levels.
Multivariate statistical analysis was performed to assess sample distribution patterns and identify markers related to beef cooking doneness. Fig. 2 displays the PCA and PLS-DA score plots, along with biplots, a 200-iteration permutation test, and VIP plots.
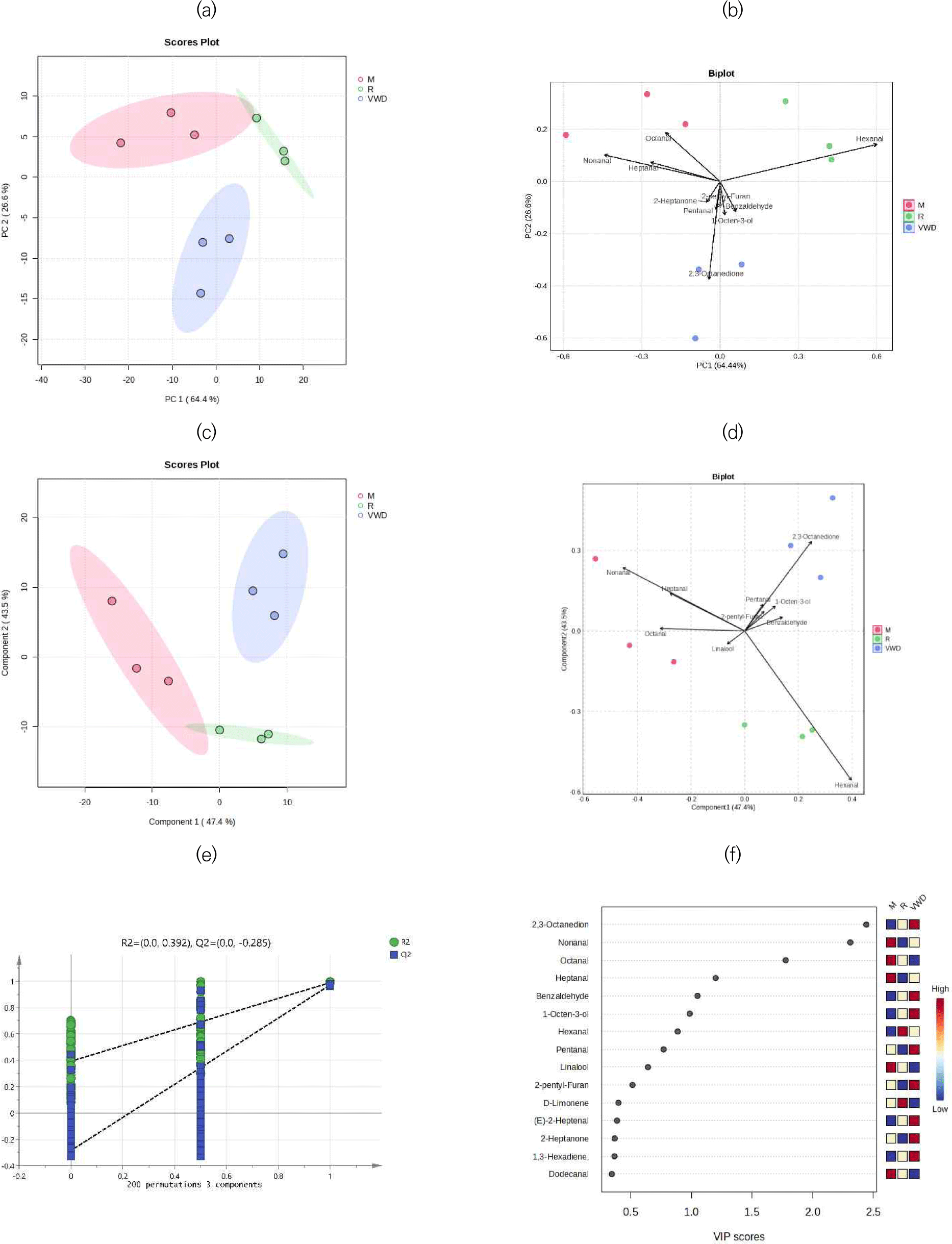
The score plot for PCA is shown in Fig. 2A, the three cooking levels (R, M, VWD) were distinctly separated along PC1 (64.44% variance) and PC2 (26.6% variance), indicating a strong influence of cooking level on the distribution of flavor compounds. The combined variance explained by PC1 and PC2 was 91.04%, demonstrating that these components effectively captured the majority of variation in flavor profiles among the groups.
As shown in Fig. 2B, the PCA biplot reveals distinct loadings of flavor compounds on PC1 and PC2. Hexanal was strongly associated with PC1, contributing to separation along this axis, while 2,3-octanedione and related compounds loaded on PC2, aiding further group differentiation. These results highlight the pivotal role of specific flavor compounds in distinguishing the three cooking levels, underscoring the influence of endpoint temperature on beef flavor profiles.
PLS-DA further extracted variables significantly contributing to cooking doneness differentiation, with results shown in Figs. 2C–f.
As shown in Fig. 2C, PLS-DA effectively discriminated beef samples across the three cooking levels. In the score plot, R, M, and VWD samples were clearly separated along Component 1 (47.4%) and Component 2 (43.5%), indicating substantial differences in flavor profiles. The ellipses around each group confirmed distinct clustering, reinforcing that cooking level significantly influenced the composition of flavor compounds.
As shown in the biplot (Fig. 2D), the direction and magnitude of flavor compound vectors reflected their contributions to sample separation. 2,3-Octanedione, 1-octen-3-ol, and benzaldehyde were closely associated with the VWD group, while hexanal was strongly correlated with the R group. Nonanal, heptanal, and octanal were prominently linked to the M group. These results demonstrate that specific flavor compounds were key in differentiating beef samples by cooking level, providing a visual basis for identifying flavor markers associated with thermal treatments.
Fig. 2E demonstrates the results of 200 permutation tests, with intercept values for R2 and Q2 at 0.392 and –0.285, respectively. These values confirm the stability of the PLS-DA model and rule out overfitting.
Fig. 2F presents the top 15 flavor compounds that most significantly contributed to the separation of the three groups in the PLS-DA analysis. Contributions are quantified using VIP scores (>1), shown on the x-axis. The colors indicate the relative concentration of each compound across the different groups. The most significant flavor compounds identified were 2,3-Octanedione, Nonanal, Octanal, Heptanal, and Benzaldehyde. Among these compounds, 2,3-octanedione is the predominant ketone in boiled beef (You et al., 2025). Wang et al. (2022) demonstrated that major aldehydes in roast beef, such as Nonanal, Octanal, Heptanal, act as markers for differentiating beef by roasting time. Benzaldehyde is a volatile Strecker aldehyde, serves as a key marker of flavor preferences in roasts and stews (Wojtasik-Kalinowska et al., 2024). In this study, the concentration of 2,3-Octanedione, Nonanal, Octanal, Heptanal, and Benzaldehyde varied with cooking doneness. Therefore, cooking doneness can be differentiated by these five potential markers.
Conclusion
Cooking doneness significantly influences the volatile flavor profile of Hanwoo beef. Higher heating intensities enhance lipid oxidation and Maillard reactions, leading to increased concentrations of key aldehydes, alcohols, ketones, furans, and sulfur compounds, particularly in VWD cooked (82°C) samples. Multivariate analyses (PCA, PLS-DA) revealed distinct separations among doneness groups and identified 2,3-Octanedione, Nonanal, Octanal, Heptanal, and Benzaldehyde as reliable markers for doneness differentiation. These findings provide a foundation for targeted flavor optimization and quality control in meat processing.
