Introduction
There is an increasing demand for functional foods on present days (Villalva et al., 2017). Probiotics are named as a functional ingredient that can beneficially affect on the human health when administered in adequate amounts. They provide plenty of benefits such as inhibition of pathogenic bacteria; lowering blood cholesterol; reduction in the incidence of constipation, diarrhea and bowel cancer; improvement of lactose intolerance, calcium absorption and vitamin synthesis; and stimulation of the immune system (Golestani and Pourahmad, 2017). The minimum recommended level of viable cell count is ranged from 106–107 but it has suggested that consuming 108–109 viable cells per day is required to obtain beneficial effects. Most common probiotic species that are used in ice cream and other dairy products are Lactobacillus and Bifidobacterium. It has been mentioned that frozen food products can maintain the stability of probiotic cultures properly compared to probiotic fermented milks (Mohammadi et al., 2011).
Therefore, ice cream could be a suitable food carrier to introduce probiotics to the human gut because it is stored at lower temperature and has a higher pH, which contribute to the survival of probiotics (Villalva et al., 2017). However, it is important to maintain the viability of probiotics throughout the storage period. Prebiotics which are non-digestible food components, contribute to the growth of probiotics and beneficially affect the host health (Di Criscio et al., 2010). Banana flour is considered as a rich source of prebiotics such as resistant starch (RS) and inulin. Moreover, banana is an abundant, low-cost ingredient found in Sri Lanka (Divisekera et al., 2019). It provides health benefits include cholesterol- and triglyceride-lowering effects, positive impact on glucose homeostasis, increasing satiety, and potential effects on the treatment of chronic kidney disease (Batista et al., 2017) and also banana is a good source of vitamin B6, potassium and vitamin C (Mahore and Shirolkar, 2018).
Blue pea (Clitoria ternatea) is an underutilized plant in Sri Lanka (Lakshan et al., 2019) which belongs to family Fabaceae and the flower petal of blue pea is a good source of dietary anthocyanin (Pasukamonset et al., 2017, Shirodkar et al., 2023) which is a plant pigment that responsible for red violet-blue color in plant flowers. The structure of anthocyanins, pH, temperature, oxygen, light and water activity are the factors that affect on the color stability of anthocyanins and it shows red color in very acidic solution and blue color in basic solution (Saptarini et al., 2015). It has been proved that anthocyanins contribute for the disease prevention and maintaining health (Shehata et al., 2020) by offering antioxidant, anti-inflammatory and anticancer properties (Náthia-Neves and Meireles, 2018).
In this study, blue pea flower (BPF) powder has been incorporated into the ice cream to act as a natural blue colorant. The aim of this study was to develop a probiotic ice cream incorporated with BPF (C. ternatea) powder and dehydrated banana flour to evaluate its sensory quality and the effect of banana flour as a potential prebiotic ingredient on the viability of probiotics (Bifidobacterium animalis subsp lactis Bb-12) during storage.
Materials and Methods
Blue colored, undamaged healthy flowers of C. ternatea L. were collected from home gardens of Badulla, Sri Lanka. Flowers were oven dried at 40°C for 24 h in drying oven (DHG-9146A, Meditry Instrument, Jiangyin, China), and ground using a domestic grinder (Xpro Duo-MG 198, Phillips, Sholinganallur, India) for 5 min, sieved (1 mm sieve) and kept in sealed airtight low density polyethelene (LDPE) bags at room temperature (Lakshan et al., 2019).
Unripened Ambun bananas at fully maturated stage were purchased from local market in Badulla, Sri Lanka. Bananas were peeled, cut into 2 mm thick slices and immediately rinsed in 2 ppm citric acid solution (Savlak et al., 2016), drained and dried at 60°C for 26 h in drying oven (DHG-9146A) until moisture content reached 10% (w/w). The dehydrated slices were ground and sieved using 60 mesh sieves (ASTM: 60; 250 μm), collected, cooled, and stored in high density polyethelene (HDPE) bags (Kumar et al., 2019).
Moisture content of blue pea powder was determined using moisture analyzer (MB25, Ohaus, Parsippany, NJ, USA).
Color was measured with a Colorimeter (B2014190, Minolta, Tokyo, Japan). The measurements were made in triplicate in a 90 mm diameter Petri dishes with a sample thickness of 20 mm, which was calibrated to measure reflectance, using the CIELAB scale for measuring the parameters [lightness (L*), red-green color (a*), and yellow-blue color (b*)] (Cui et al., 2021).
Total anthocyanin content of BPF powder were analyzed according to the pH differential method (Lee et al., 2005) as follows. Briefly, 1 g of blue pea powder was transferred into 10 mL volumetric flask for extracting and preparing two dilutions of the sample, one was potassium chloride buffer at pH 1.0 and the other was sodium acetate buffer at pH 4.5. The volumes were fixed by diluting each. It was allowed these dilutions equilibrate for 15 min. The absorbance of each dilution was measured at the 510 and 700 nm (to correct for haze), against a blank cell filled with distilled water using UV spectrophotometer (4120020, J.P. Selecta, Barcelona, Spain). All measurements should be between 15 min and 1 h. The longer standing times spend after sample preparation could contribute in increasing the readings. Absorbance readings were made against water blanks. The samples to be measured were clear and contain no haze or sediments; however, some colloidal materials might be suspended in the sample, causing scattering of light and a cloudy appearance (haze). This scattering should be corrected by reading at a wavelength where no absorbance of the sample occurs, i.e., 700 nm. The following formula was used to calculate the absorbance of the diluted sample (A).
Monomeric anthocyanin pigment concentration in the original sample were calculated using the following formula:
Where the molecular weight is abbreviated by MW, dilution factor is abbreviated by DF, and the molar absorptivity is indicated as ε, cyanidin-3-glucoside was used for calculating pigment content (MW=449.2 and ε=26,900) (Sutharut and Sudarat, 2012).
Banana flour was chemically evaluated for moisture, protein, fat, ash and fiber (AOAC, 2016).
Total soluble carbohydrates in prepared dehydrated banana flour were determined using phenol-sulfuric method (DuBois et al., 1956) using pure glucose as a standard, and reported as glucose equivalents.
Enzymatic spectrophotometric method was used to determine the inulin content in dehydrated banana flour. Inulin contents of prepared dehydrated banana flour were determined following the enzymatic spectrophotometric method and utilizing the Megazyme fructan assay kit. Inulin was extracted by dissolving 1 g of dehydrated banana flour in 100 mL of hot Milli-Q water at 85°C for 20 min with continuous stirring and then filtering through a Whatman No. 1 filter paper (Maidstone, UK).
The inulin content in the extract was determined using the fructan assay kit. The inulin content of each banana flour was calculated using the following formula:
Where _A=sample absorbance–sample blank absorbance (both read against the reagent blank), F=factor to convert absorbance values to μg of fructose [(54.5 μg of d-fructose)/(absorbance for 54.5 μg d-fructose)], V=volume (mL) of extract used, and W=weight (mg) of sample extracted (Mudannayake et al., 2015).
Validation of the method of analysis was established using standardfructan samples (which contained 25.5% inulin; Megazyme International, Bray, Ireland) (Mudannayake et al., 2015).
Ingredients required for the preparation of ice cream were purchased from a retail store in Badulla, Sri Lanka. For the preparation of ice cream mix, 1 L of fresh milk, full cream milk powder (FCMP), cream fat, white sugar, liquid glucose, stabilizer, gelatin and BPF powder were added at 4%, 8%, 24%, 2%, 0.4%, 0.2%, and 0.67% (w/v) composition, respectively. Table 1 shows the composition of four treatments of ice cream.
| Treatments | BF% (w/w) used to replace FCMP (%) |
|---|---|
| T1 | 0 |
| T2 | 10 |
| T3 | 20 |
| T4 | 30 |
The mix was pasteurized (85°C for 30 s) and cooled down to 70°C and homogenized in a homogenizer (FT9, Armfield, Ringwood, England) for 150 Pa. Then 0.22% (w/v) of vanilla flavor was added at room temperature and the mix was kept in 4°C for 24 h in refrigerator (RT33FAJFASL, Samsung, Seoul, Korea) for ageing. After ageing, mix was warmed-up to inoculate probiotic culture at fermentation temperature (40°C). Then freeze-dried single strain B. animalis (Bb-12®, Chr Hansen, Hoersholm, Denmark) was added and incubation was done until pH 5.8 at 42°C. After incubation in an incubator (JRIC-10, Osworld, Mumbai, India), mix was cooled down to 4°C and sent to the ice cream machine (YKF 826, Eastman, Shanghai, China) to improve overrun and hardening. Finally, ice cream was stored in a freezer (TX-350L, WESTPOINT, Shanghai, China) in –20°C (Mohammadi et al., 2011).
The design of the sensory evaluation conducted in this study was ethically reviewed and approved by the Research Ethics Committee of Uva Wellassa University (No. UWU/REC/2023/ 08). Sensory quality (appearance, color, aroma, texture, taste, mouthfeel, overall acceptability) of treatments was analyzed under seven-point hedonic scale (1=dislike very much and 7=like very much), using 30 untrained panelists, both male and female, aged 21–30. The evaluation was conducted in the panel booths in accordance with the standard regulations at the university sensory laboratory (Granato et al., 2012).
The following analyzes were performed on the four treatments on day 1, day 7, day 14 and day 21 during storage: pH, color, and Brix values were measured (Cui et al., 2021) using pH meter (MP511, TBT, Nanjing, China), colorimeter (B2014190) and refractometer (2312-E07, ATAGO, Tokyo, Japan), respectively. Proximate analysis of final product were carried out to determine total solids, ash, fat and protein (AOAC, 2016).
Melting rate was determined as follows; whereby 25 g of the product were placed on a metal mesh with 0.7 mm diameter openings and allowed to stand at 20±2°C. The values obtained were recorded with a digital timer and expressed in seconds (Villalva et al., 2017).
Counts of B. animalis subsp lactis Bb-12 were enumerated according to the spread plate technique using Beerens agar (Beerens, 1990). The incubation was done in anaerobic conditions established in anaerobic jars using anaerobic atmosphere generating sachets (AN0035A, Thermo Scientific, Basingstoke, UK) at 37°C for 72 h. Viable Bifidobacterium lactis cell count was performed on days 1, 7, 14, and 21 during storage.
All the experiments were conducted in triplicate. Sensory evaluation data were analyzed by using Friedman test. The data was analyzed using completely randomized design, one-way analysis of variance using MINITAB 17 software and the differences between treatment groups were determined using Tukey test.
Results and Discussion
Moisture content was determined as 11.10±0.02% (w/w) and total anthocyanin content in BPF powder as measured by pH differential method was 1,168.92 ppm. Color of BPF powder was determined as 34.71±0.16 L*, 4.08±0.08 a* value and –2.67±0.02 b* value. Previous study noted that 190 mg/100 g and 52 mg/100 g anthocyanin content were found in wild blueberry juice concentrate and puree, respectively (Camire et al., 2006). Total anthocyanin content resulted for BPF powder in the current study was 23,378.4 mg/100 g (1,168.92 ppm). Therefore, BPF powder has a higher value of total anthocyanin content, and it means BPF powder is more concentrated with anthocyanin which gives extreme blue color. Moreover, it has been mentioned 11.72 L*, –0.06 a*, –0.20 b* values for juice concentrate and 3.66 L*, 10.09 a*, 1.53 b* values for puree which has lower number of anthocyanins than the concentrate. Therefore, resulted color values for blue pea in this study had been higher L* value than wild blueberry concentrate and puree, higher a* value than concentrate but lower a* value than puree and lower b* value than concentrate and puree. These changes of color would be caused due to the differences in water activity and pH of above three anthocyanin sources because Wrolstad (2004) revealed that anthocyanins have been increased stability at reduced water activity, which is made them suitable for dried and intermediate moisture foods and also anthocyanins reversibly experience structural transformation with change in pH.
Table 2 lists the chemical composition obtained by chemical analysis of dehydrated banana flour.
Previous study of Sardá et al. (2016) mentioned that unripen banana contains high content of RS and a low content of soluble sugar. Therefore, it is considered to be a source of RS which beneficially affect on human health. Menezes et al. (2011) reported that unripened banana flour, which are produced under specific conditions were comprised as follows: 73.4 g/100 g total starch, 17.5 g/100 g RS and 14.5 g/100 g dietary fiber content and moreover, it suggested that producing unripened banana flour with high RS content and considering it a functional ingredient requires the differentiation of the stages of banana ripeness.
Inulin content in dehydrated banana flour in this study was 0.87±0.16 g/100 g. When considering the amount of inulin in other food sources, chicory root has been reported as the most concentrated source of inulin and oligofructose. The content of inulin in banana was analyzed by various researchers. The inulin reported in dried raw banana was 1.4±0.6 g/100 g as mentioned in Moshfegh et al. (1999), 0.40 g/100 g and 0.06 g/100 g amount of inulin were found in common ripe and unripe banana, respectively as reported by Judprasong et al. (2011). According to Shalini and Antony (2015), inulin content in Hill banana, Karpooravalli, Nendran and Rasthali cultivars were; 0.23, 0.17, 0.85, and 0.19 (g/100 g), respectively. Furthermore, it showed that Morris, Poovan and Red banana cultivars had not been detected with inulin. Moreover, Pongmalai and Devahastin (2020) indicated that inulin content in Nendran banana were increased during ripening due to the accumulation of these carbohydrate polymers. However, in contrast the inulin content had been reported less than 1 g/100 g in banana according to the previous studies.
The results obtained in this research was in agreement with the reported results in Judprasong et al. (2011), Moshfegh et al. (1999), and in Shalini and Antony (2015). It was apparent that banana is not a rich source of inulin, even though it is a rich source of RS and fiber.
Fig. 1 shows the mean values of sensory analysis conducted for probiotic ice cream samples with different banana flour percentages. Ice cream which had been incorporated 20% of BF showed significantly higher (p<0.05) mean values for appearance, color, texture, aroma, taste, mouthfeel and overall acceptability. Normally, incorporating prebiotic ingredients into ice cream may occur a greater influence on flavor and texture but metabolism of probiotic culture can result components in product that may adversely affect on taste and aroma (Mohammadi et al., 2011). However, neither probiotic culture nor banana flour negatively affected on aroma and taste of the ice cream in the study.
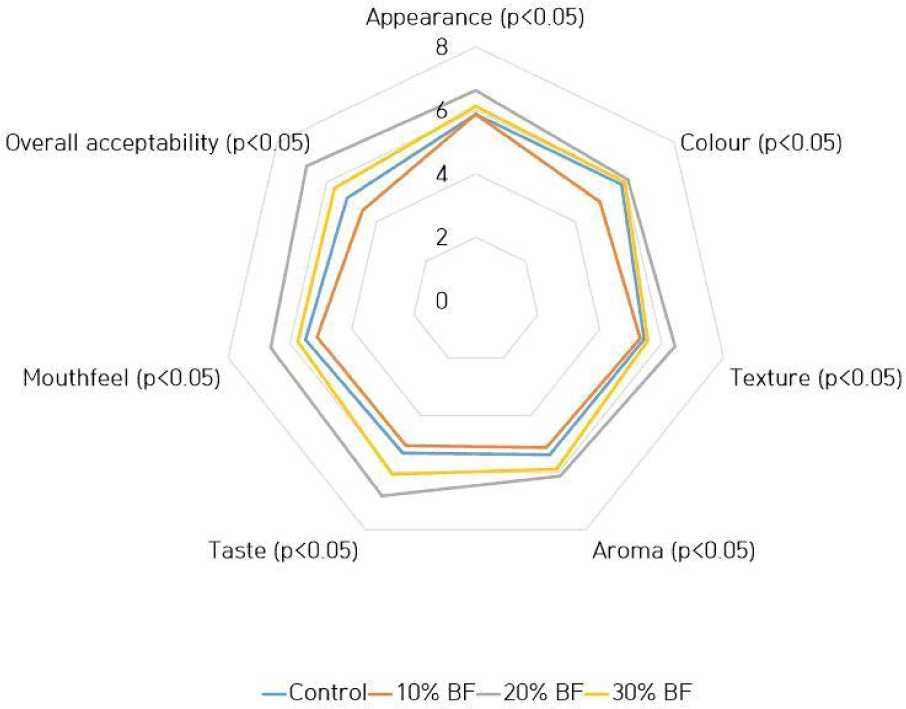
Table 3 shows the changing of pH values of different treatments with the storage time. There were significant differences (p<0.05) in pH values among four treatments and also among the days of storage. But it doesn’t show a pattern in fluctuating the pH values. Therefore, that kind of result could be obtained due to the results of incubation process, differences of composition in banana flour and the existing live microbial count in the ice cream samples. Fig. 2 illustrates the color (L*, a*, b*) values of treatments, that are changed with the storage. It shows that there is a significant difference (p<0.05) among the ice cream samples and also among the days of storage. Color of the ice cream samples could be changed due to the presence of anthocyanins in the blue pea powder which is sensitive to pH (Lakshan et al., 2019).
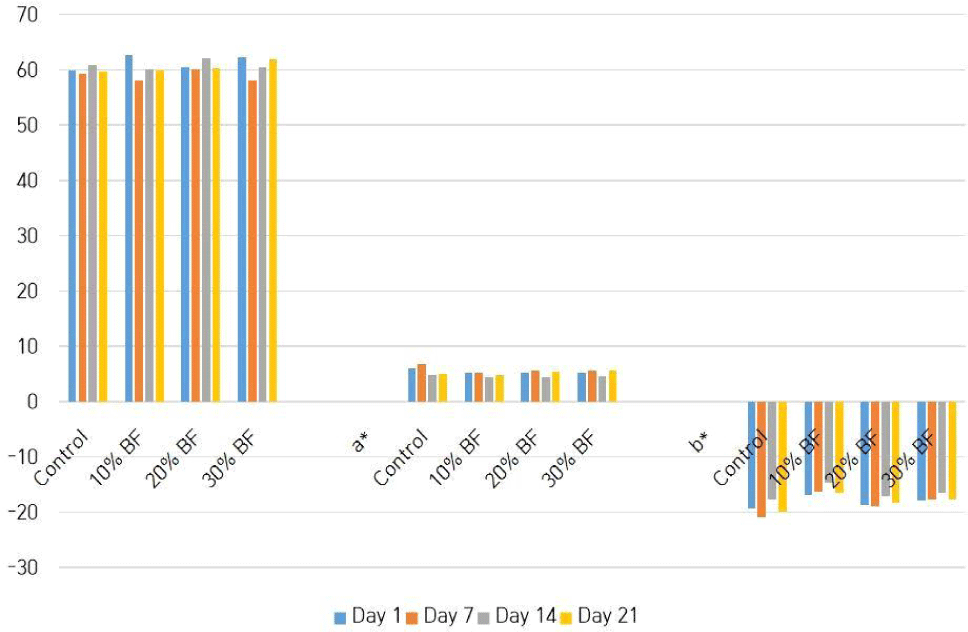
Table 4 shows the counts of probiotic bacteria during storage. It was found no significant differences (p>0.05) in colony counts among the days of storage. The viability of probiotics was remained >109 CFU/g in every sample during the time period of storage and it is a high value compared with the minimum recommended level (106–107).
The nature of probiotic populations generally reveals good survivability in ice cream throughout their shelf life. During storage, variations in the survival time of probiotic bacteria depend on the strain, the production technology, storage temperature, storage time and product formulation (Mohammadi et al., 2011). Bifidobacterium are anaerobic bacteria, therefore molecular oxygen as well as high values of redox potential would be critical factors for their survival (Villalva et al., 2017), but it was found that survival of Bifidobacterium in ice cream over 70 days of frozen storage was approximately 90% and it has been suggested that ice cream is an excellent vehicle for delivering Bifidobacteria into the human diet (Hekmat and McMahon, 1992).
The addition of prebiotic compounds can significantly improve the retention of probiotic viability (Mohammadi et al., 2011). Probiotics or specific populations of the resident microbiota selectively metabolize prebiotics, such as inulin and enhance their growth and/or activity in the large intestine. Thus, prebiotics can be added to probiotic products as crucial growth factors and could considerably improve cells viability (especially for Bifidobacteria) (Villalva et al., 2017). According to the results shown in Table 4, there are no significant differences (p>0.05) among four treatments of ice cream samples. Therefore, it indicates that percentages of banana flour used in the study had not been significantly influenced the viability of probiotics as a prebiotic. Moreover, it can be explained by the fact that banana flour contained only low quantity of inulin in them (0.87±0.16 g/100 g), thus was not capable of supporting the probiotic survival in the product compare to the control.
Conclusion
This study revealed that banana flour did not significantly improve the survival of probiotic as a prebiotic source, however, significantly increased the sensory quality of the ice cream. The results proved that banana is a not a significant source of inulin as it only containing less than 1% inulin/100 g on its dry basis. Nevertheless, it was not found any significant reduction in live Bifidobacteria in the ice cream throughout the storage period. Probiotic ice cream incorporated with BPF (C. ternatea) and dehydrated banana flour proved to have excellent organoleptic characteristics that would enhance consumers acceptability.
