Introduction
Lycopene is the principle carotenoid responsible for the red pigment of tomato (Shi, 2000). Sharma and Le Maguer (1996) found that the peel of tomato is a rich source of lycopene, as they contained about five times more lycopene than the whole tomato pulp. Lycopene, with its acyclic structure, large array of conjugated double bonds, and extreme hydrophobicity, exhibits many unique and distinct biological properties, including an antioxidant, anti-carcinogenic, strong color and non-toxicity (Jiang et al., 2016; Mein et al., 2008; Papaioannou et al., 2016; Rizk et al., 2014; Stahl and Sies, 2005). Lycopene is the most efficient singlet oxygen quenchers of the natural carotenoids (Papaioannou et al., 2016; Stahl and Sies, 2007). The antioxidant activities of lycopene are highlighted by their singlet oxygen quenching properties and their ability to trap peroxyl radicals (Choe and Min, 2009; Mein et al., 2008; Stahl and Sies, 2007). Lycopene is also considered as a natural pigment that is highly accepted by food industry as a natural colorant. Rizk et al. (2014) showed the use of carotenoids extracted from tomato peel as a natural colorant and antioxidant in ice cream. The peel fraction of tomatoes (a waste product of tomato industries) could be used to produce functional food ingredient in other food products where it could play an important role in improving antioxidant intake in the human diet.
Lycopene contains numerous, proven health benefits including; preventing and retarding cancers, cardiovascular diseases, coronary heart disease and oxidative stress (Assar et al., 2016; Gupta et al., 2003; Muller et al., 2016; Rao and Rao, 2007; Shi and Le Maguer, 2000). Recently, there is an emerging interest on natural ingredients in reducing the risk of communicable and non-communicable diseases. Therefore, producing functional foods which contain natural health ingredients are gaining more popularity than regular food products among the health conscious consumers. Direct addition of the lyophilized tomato peel powder into stirred yoghurt could be more advantageous than adding of isolated lycopene, as the isolation of lycopene is a challenging and an expensive process.
Yoghurt is one of the most popular dairy products which is well-known for its health benefits (Walstra et al., 2005). This study was focused on development of a functional stirred yoghurt with high antioxidant activity, incorporating tomato peel powder as a source of lycopene.
Materials and Methods
Ripened tomatoes (variety Thilina) were harvested from local fields in Badulla, Sri Lanka. Tomatoes were cleaned, immersed in boiling water for 1–2 minutes, cooled under tap water and hand peeled. The peels were frozen at −18°C in a freezer for two days (TX-350L, Westpoint, Guangdong, China), followed by freeze drying in a freeze dryer (FD5512, ilShinBioBase, Dongducheon, Korea). The freeze dried tomato peels were ground, passed through 0.70 mm sieves and transferred into air tight containers and stored at −20°C until used.
Carotenoid (lycopene) was extracted using the method described by Hackett et al. (2004). The oily fraction of carotenoids (lycopene) extracted from Tomato Peel Powder (TPP) was transferred into a dark bottle and freezed (−20°C) until further analysis.
Total carotenoid (lycopene) content was determined according to the method described by Strati and Oreopoulou (2010). The extract was centrifuged at 1,966×g for 10 minute (Sorvallst 40R, Thermo Fisher Scientific, Dreieich, Germany) to separate the supernatant. The carotenoid content of the supernatant was measured using spectrophotometer (Basic, Eppendorf, Hamurg, Germany) at 471 nm and expressed as lycopene. Hexane was used as the blank. The following equation was used to calculate the carotenoid concentration (C), expressed in lycopene equivalent (mg L−1), in extract;
Where A is the absorbance at 471 nm and is the absorption coefficient of lycopene (absorbance at the maximum wavelength of a 1% solution in a spectrophotometer cuvette with 1-cm light path) of 3,450 in hexane.
The extraction yield of lycopene (LY) was calculated by the equation (2):
Where C is the concentration of lycopene in the solvent (mg L−1), calculated by equation (1), V is the volume of the extract (L) and W is the dry weight of tomato waste used in the first extraction (kg) (Strati and Oreopoulou, 2010).
Total phenolic content was determined using Folin-Ciocalteu’s reagent method as described by Thomas and Wansapala (2017) using a concentration series of gallic acid (5–250 ppm). The absorbance was measured with a UV–Vis Spectrophotometer (Basic, Eppendorf) at 765 nm against the blank. Results were expressed as mg of gallic acid equivalent per gram of sample (mg GAE g−1).
Radical scavenging activity (RSA) of TPP and commercial lycopene (HerbaDiet®, 10% lycopene) was measured using DPPH (2, 2-diphenyl-1-picylhydrazyl) method as described Shimada et al. (1992) and expressed as percentage inhibition of the DPPH radical. The ability to scavenge the DPPH radical was calculated using the following equation.
Chemical characterization of lyophilized TPP, extracted lycopene and commercial lycopene (10% lycopene, HerbaDiet®, Rohtak, India) were assessed using FTIR spectroscopy (ALPHA spectrophotometer, Bruker, Karlsruhe, Germany) and OPUS 7.5 FTIR software against a KBr background. The FTIR spectra were collected at the wavenumber range 400–4,000 cm−1 at a resolution of 4 cm−1 and 32 scans for each spectrum (Bunghez et al., 2011).
UV-visible (UV-VIS) spectral analysis was performed for lyophilized TPP, extracted lycopene and commercial lycopene (10% lycopene, HerbaDiet®) using UV-VIS Spectrophotometer (Basic, Eppendorf). Tomato peel powder (1 g), extracted lycopene of TPP (1 g) and commercial lycopene (1 g) each was dissolved in 5 mL of hexane separately and centrifuged for 3,494×g for 5 min. Supernatant of each sample was used for UV-VIS spectral analysis at a range of wave length from 400–550 nm (Bunghez et al., 2011).
Tomato peel extract was examined for yeast according to method described in Kiros et al. (2016) with slight modification.
Two batches of stirred yoghurts were prepared incorporating TPP before fermentation and after fermentation according to the method described by Dias and Jayasooriya (2017) with slight modifications. First batch of “before fermentation” samples were prepared by mixing different percentage levels of pre homogenized standardized fresh milk with, same levels of sugar (6.5%, w/w), gelatins (0.25%, w/w) and lyophilized TPP at concentrations of 0%, 2%, 4%, 6%, and 8% (w/w) at 60°C to make up the final weight as 100% (w/w). Then each sample mixture was pasteurized at 90°C for 10 min and cooled to 44°C. Milk mixtures were inoculated with commercial yoghurt culture (0.02%, w/w) which containing Streptococcus thermophiles, and incubated at 42°C for 4 h. Upon incubation, the coagulum of each yoghurt was broken by gentle stirring and divided into pre labeled yoghurt cartons. The prepared stirred yoghurts were stored at refrigerator (4°C±2°C) for further analysis. The second batch of stirred yoghurt samples were prepared same as above, except that different levels of lyophilized TPP (0%, 2%, 4%, 6%, and 8% (w/w)) was added after fermentation, at the time of breaking the coagulum.
Sensory analysis of stirred yoghurts which incorporated with TPP “before fermentation” and “after fermentation” were carried out using 9 point hedonic scale (1=dislike extremely and 9=like extremely), using 30 untrained panelists. Temperature and the light were kept constant in the sensory room throughout the sensory evaluation period.
Free RSA (%) of the prepared stirred yoghurts was measured at 7 days interval for refrigerated storage (4°C±2°C) of 21 days using the method of Shimada et al. (1992) and expressed as percentage inhibition of the DPPH radical. Five hundred mg of yoghurt from each sample were dissolved in 10 mL methanol separately. The solutions were vortexed for 1 min and centrifuged at 3,494×g for 5 min to obtain a clear solution. The supernatant of each yoghurt sample was used to analyze the RSA using the method described as above for tomato peel.
Color of all ten stirred yoghurt samples incorporated with different levels of TPP was (0%–8% levels) investigated at 7 days interval at refrigerated storage for 21 days using colorimeter (B2014190, Minolta, Tyoko, Japan).
Stirred yoghurts incorporated with TPP were examined for coliform/ Escherichia coli, yeast and molds by following standard AOAC methods (2016).
The pH of each stirred yoghurt sample incorporated with TPP was determined at 1, 7, 14, and 21 days of refrigerated storage (4°C±2°C) according to the method described in AOAC 947.05 (1990). Crude fat, moisture, ash and total solid content of the product were analyzed at day 1 described in AOAC (1995).
Results and Discussion
The total lycopene content (yield) of TPP was 7.14±0.01 mg 100 g−1 (dry weight basis), which was comparable to that of revealed by Toor and Savage (2005) in tomato peels (8.7±1.12 mg 100 g−1). Al-Wandawi et al. (1985) reported that the lycopene content of tomato skin is 12 mg 100 g−1, while George et al. (2004) reported that the lycopene content of tomato skin is ranged from 5–14 mg 100 g−1. The result of the present study lies within the range of the results reported in previous studies.
Tomato peel powder extract showed a phenolic content of 0.38±0.01 mg GAE g−1. Toor and Savage (2005) studied that the phenolics in the skin of tomatoes in different cultivars ranged from 0.26–0.30 mg GAE g−1. Further, George et al. (2004) reported that the phenolic content in the skin of tomatoes grown in different fields ranged from 0.10–0.40 mg catechin equivalents g−1. The phenolic content of the tomato peel of the present study was within the range of previous researchers’ results.
Radical scavenging activities (%) of tomato peel powder and commercial lycopene were 50.05±0.66% and 45.32±0.22%, respectively. RSA of the tomato peel powder was found to be higher than commercial lycopene. Such variation in results can be due to storage period and conditions of storage of samples which may have led to partial deterioration of the lycopene. Elbadrawy and Sello (2016) reported that RSA of dry tomato peels obtained from Egypt was 86.4%. The result of present study has been less than that found in previous research. The different values of RSA of tomato peel are apparently due to the variations in cultivars, climatic conditions, growing conditions, stage of ripening and methods used in analysis.
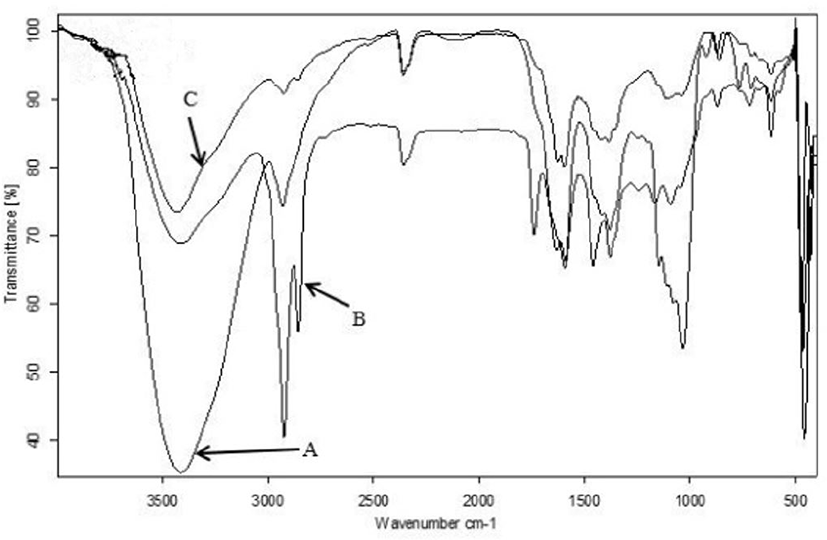
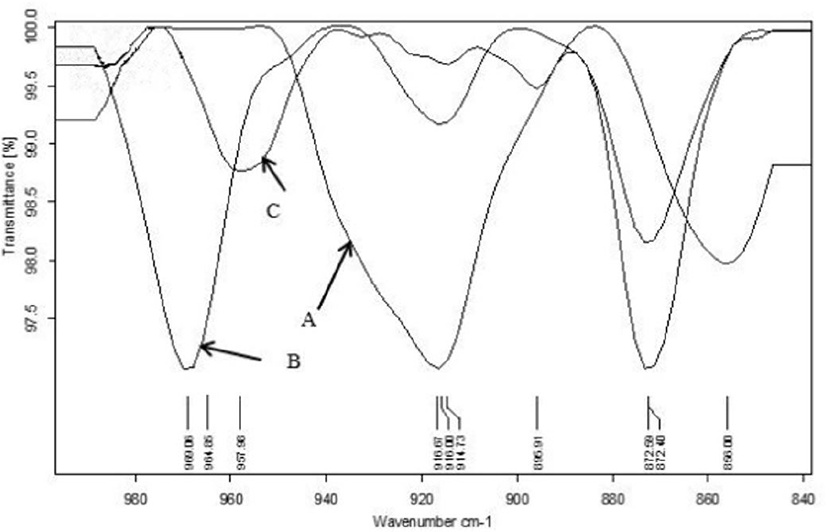
The FTIR spectra (Fig. 1) of commercial lycopene, extracted lycopene of TPP and TPP showed following functional groups, as OH stretching at 3,428.48–3,412.58 cm−1 (Bunghez et al., 2011), and vibrational C=C, CH2 symmetrical and asymmetrical stretching at 2,931.36–2,855.22 cm−1 (Irudayaraj and Tewari, 2003). Other bands occur at 1,632.96–1,582.65 cm−1 (amide), 1,384.66–1,379.31 cm−1 (CH3 bending) and 1,044.88–1,032.64 cm−1 for C-O stretching (Bunghez et al., 2011; Kalaivani, 2015; Kamil et al., 2011). The frequency region between 900–670 cm−1 show bands attributed to C-H bends stretch alkene (Kamil et al., 2011). The spectral bands obtained at a frequency of 960–957 cm−1 can be attributed to the presence of trans CH of lycopene (Fig. 2) found in tomatoes (Kamil et al., 2011; Williams and Norris, 2001).
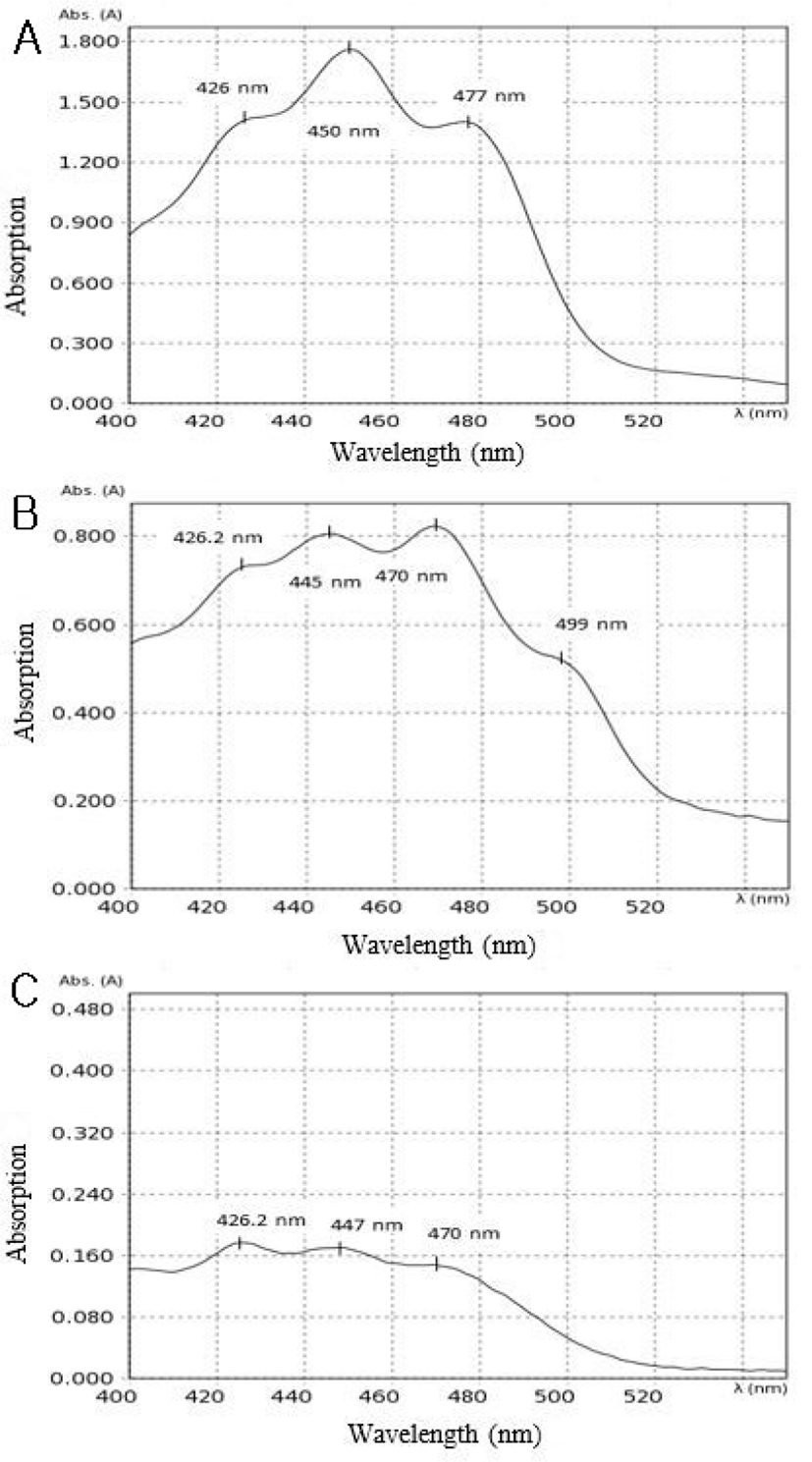
The results of the UV-VIS spectral analysis of commercial lycopene, extracted lycopene of TPP and TPP are shown in Fig. 3. The UV-VIS spectrum of lycopene in literature shows the characteristic bands of lycopene at 447.2 nm, 457 nm, 470 nm and 500 nm (Aghel et al., 2011; Bunghez et al., 2011; Chemat-Djenni et al., 2010; Moigradean et al., 2007). These characteristic bands of lycopene were also shown in commercial lycopene (Fig. 3A), extracted lycopene of TPP (Fig. 3B) and TPP (Fig. 3C). The band located at 426.2 nm is responsible for the alpha-carotene (Chemat-Djenni et al., 2010). UV-VIS spectral results confirmed that presence of lycopene, in laboratory prepared tomato peel powder.
E. coli, yeast and molds were not detected in tomato peel powder samples. The initial blanching, hygienic handling of tomatoes and quick reduction of moisture content by freeze drying under the laboratory conditions, may have led to such high microbiological quality of TPP.
Results of RSA (Table 1) showed that stirred yoghurts incorporated with TPP had significantly higher (p<0.05) RSA compared to the control (no TPP). As well as RSA was directly proportional to the percentage of TPP incorporated into yoghurt samples. At the day one, the highest RSA (23.07±0.45%) was shown by stirred yoghurt, which was prepared with 8% (w/w) TPP, added “after fermentation”, while the least value (1.58± 0.10%) was shown by control samples, which had no TPP incorporated (Table 1).
RSA was decreased with the increase in storage time as shown in the Table 1. For example, the RSA of stirred yoghurt incorporated with 8% (w/w) TPP, after fermentation, was 23.07±0.45%, while RSA of the same sample at day 21 was 15.30±0.17%. Here, lycopene is in contact with yoghurt, bacteria, other compounds and dissolve oxygen. The deterioration of RSA is presumably due to various oxidative mechanisms. These results are in agreement with Rizk et al. (2014) who found that RSA of tomato peel extract (lycopene) incorporated ice cream. Stirred yoghurt prepared by incorporating 8% TPP after fermentation, showed significantly higher RSA values (23.07±0.45%) compared to 8% TPP added before fermentation samples (16.83±0.36%). According to the D’Evoli et al. (2013); Mayeaux et al. (2006); Shi (2000), the lycopene in tomato peel can be destructed due to the effect of heat. During the production of stirred yoghurts, milk is subjected to super pasteurization (90°C for 10 min). The lesser value of RSA of tomato peel powder incorporated stirred yoghurts, “before fermentation” is apparently due to the partial destruction of lycopene in tomato peel powder during super pasteurization process.
Table 2 and Table 3 illustrate the color of stirred yoghurts fortified with TPP “before” and “after fermentation” according to the three stimulus color coordinates (L value-lightness, a value-redness and b value-yellowness). The addition of TPP has significantly (p<0.05) increased all the color parameters of the stirred yoghurts. The lowest value for lightness and highest value for redness and yellowness were obtained by stirred yoghurts fortified with 8% (w/w) TPP compared to the control yoghurt samples. Increase in tomato peel powder concentration (from 2%–8%) has significantly increased ‘a’ value and ‘b’ value in stirred yoghurts while “L” value was decreased. According to the results, the color of the stirred yoghurts was not significantly (p>0.05) affected by storage time.
L value, 0=black and 100=white; a value, −60=green and +60=red; b value, −60=blue and +60=yellow. Data represented as mean±SD (n=3).
L value, 0=black and 100=white; a value, −60=green and +60=red; b value, −60=blue and +60=yellow. Data represented as mean±SE (n=3).
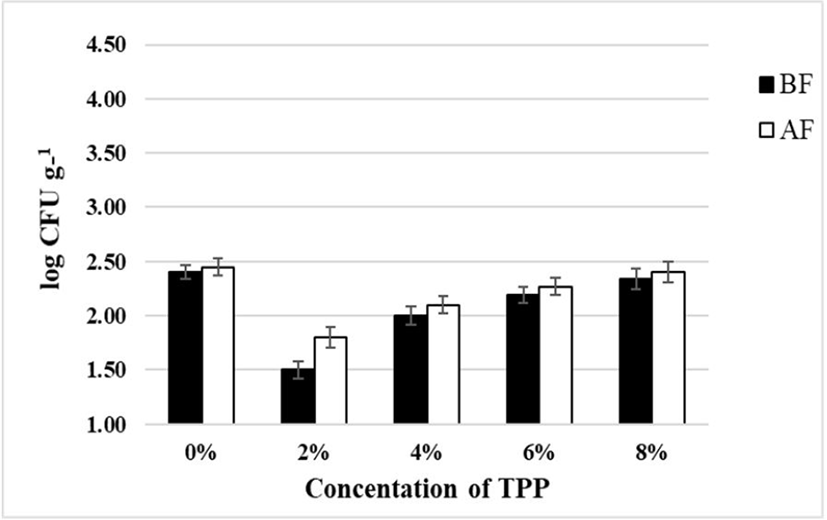
E. coli was not detected in stirred yoghurts fortified with TPP “before fermentation” and “after fermentation” at day 1 and day 21 (data not shown). Yeasts were not detected at day 1 for both samples prepared “before fermentation” and “after fermentation”, however, detected at 21 days of refrigeration in both samples (Fig. 4).
Results of Chemical Analysis
During the refrigerated storage period of 21 days, the pH of stirred yoghurts was decreased gradually in all samples (Table 4). Storage temperature and type of culture used for yoghurt are main factors affecting the pH variation of stirred yoghurts during storage (Thomas and Wansapala, 2017). The pH was decreased during storage period due to the production of lactic acid by live starter bacteria present in the yoghurt.
According to the results shown in Table 5, crude fat content of all samples was not significantly different (p>0.05) from each other. Stirred yoghurts incorporated with TPP (2%, 4%, 6%, and 8% w/w) contained higher ash and total solid content compared to the control group which was prepared with 0% (w/w) TPP. The moisture content of the stirred yoghurts incorporated with TPP was lower than that of control group.
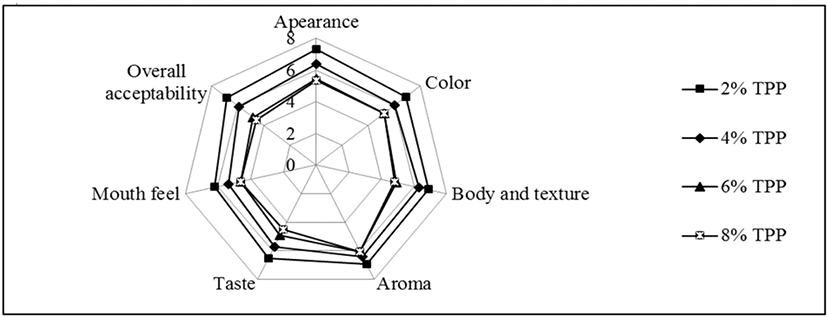
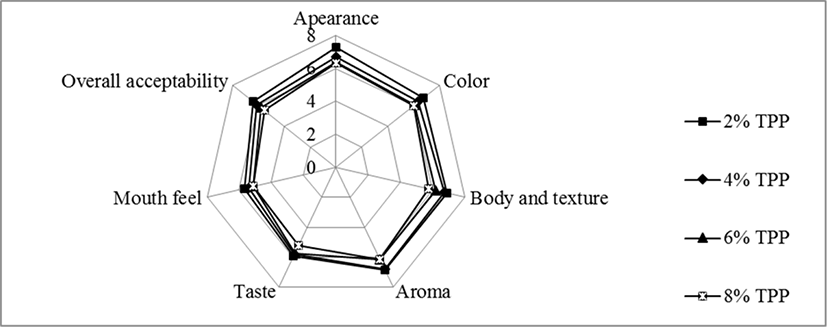
Highest estimated median was obtained by stirred yoghurt which containing 2% of TPP (except the control group) for all sensory attributes (appearance, color, body and texture, aroma, taste, mouth feel and overall acceptability) for both before and after fermentation while the lowest estimated median was obtained by 8% TPP incorporated stirred yoghurt. The results of the sensory analysis of stirred yoghurts incorporated with TPP “before” and “after fermentation” were illustrated as web diagrams (Fig. 5 and 6). The results of the sensory evaluation, showed that 2% tomato peel powder incorporated stirred yoghurt was perceived as the best product by the panelists in terms of the all the sensory attributes.
Conclusion
Results of the study revealed that lycopene of tomato peel can be utilized as an active natural antioxidant agent and a colorant in stirred yoghurt. The addition of 8% tomato peel powder appeared to improve antioxidant activity and color significantly. However, a lower concentration of tomato peel powder (2%) was preferred by consumers than the higher levels. Results suggest that commercialization of these types of products must find a balance between consumer liking and maintaining therapeutic amounts of antioxidant ingredients.
