Introduction
Based on its simple anatomy, known genome sequence, and similar metabolic pathways compared to mammals, Drosophilamelanogaster can be a useful model to evaluate the health effects of food components, including on reproduction and developmental processes (Pandey and Nichols, 2011). The reproduction process of D. melanogaster occurs in the sequence of courtship, mating, oogenesis, and ovulation. It is known that polyunsaturated fatty acids (PUFA) exhibit a diverse range of critical functions in reproduction, such as the biosynthesis of hydrocarbons, formation of lipid signaling molecules (e.g., eicosanoids), and lipid deposition during oogenesis (Vrablik and Watts, 2013), which can influence reproduction process of D. melanogaster (Marcillac et al., 2005; Ueyama et al., 2005). In particular, the hydrocarbons 7,11-heptacosadiene and 7,11-nonacosadiene, derived from PUFA on female cuticles, function as the principle female sex pheromones to stimulate male courtship (Antony et al., 1985; Yew et al., 2009) and lead to increased reproduction ability (Chen and Buhler, 1970; Savarit et al., 1999).
Conjugated linoleic acid (CLA), a group of C18:2 fatty acids with conjugated double bonds, has been regarded as a functional food ingredient, especially for its role in lipid metabolism (Kim et al., 2016). Studies in rodents and humans, as well as Drosophila, reported reduced body fat with elevated lean body mass after CLA-supplementation (Chen et al., 2018; Kim et al., 2016; Park et al., 1997). Current knowledge on the effects of CLA on development and reproduction is limited and inconsistent. CLA decreased egg production rate and increased embryo mortality in hens and quails (Aydin and Cook, 2004; Leone et al., 2009), which was suggested to be associated with changes in fatty acid composition in egg yolk by CLA; yolks had higher saturated fatty acid and lower monounsaturated fatty acid due to the down-regulation of stearoyl-CoA desaturase (Chamruspollert and Sell, 1999; Lee et al., 1998). In addition, CLA influences proteins associated with biological processes of reproduction and development in Caenorhabditis elegans, although it did not significantly affect egg laying and hatchability in C. elegans (Shen et al., 2018). Another publication reported that CLA decreased larval development and survival rates of European corn borer, Ostrinia nubilalis (Gereszek et al., 2008). However, no other adverse effects by CLA were observed on the adult survival, fertility, larval development, and the eclosion of oviposited eggs in a house fly study (Park et al., 2000). Thus, we aimed to determine the effects of CLA on reproduction and development using D. melanogaster.
Materials and Methods
Drosophila stock bottles, vials, grape agar food, and embryo collection cages were purchased from Genesee Scientific (San Diego, CA, USA). Clarinol G-80 Kosher (composed of triglyceride (TG) form of CLA with 34% of cis-9, trans-11, 35% of trans-10, cis-12, and 5% of other CLA isomers, as well as 2% of linoleic, 6% of palmitic, 3% of stearic, and 13% of oleic acids) was from Lipid Nutrition B.V. (Channahon, IL, USA). Safflower oil (composed of 69% of linoleic, 7% of palmitic, 3% of stearic, and 19% of oleic acids) was from California Oil Corporation (Richmond, CA, USA). Standards of 7,11-heptacosadiene (7,11-HD) and 7,11-nonacosadiene (7,11-ND) were purchased from Cayman Chemical (Ann Arbor, MI, USA). 1-Docosene was purchased from Combi-Blocks (San Diego, CA, USA) as an internal standard. High Capacity cDNA Reverse Transcription Kit and StepOnePlus Real-Time PCR System were purchased from Applied Biosystems (Foster City, CA, USA). Other chemicals, reagents, assay kits, and primers were purchased from Thermo Fisher Scientific (Waltham, MA, USA) unless stated otherwise.
The Canton-S strain was used for all experiments. Flies were reared on Jazz-Mix Drosophila food in a 12:12 h light/dark cycle at 25°C in a DigiTherm® CircKinetics™ incubator (Tritech Research, Los Angeles, CA, USA). One to two days old of flies were anesthetized with CO2 before transferring to experimental diets. The experimental diets were prepared by adding 0.5% (w/v) water (as blank), CLA, or safflower oil (as LA control) to the standard fly food. The concentration of oils was chosen based on the previous publications showing that 0.5% of CLA is effective for body composition regulation in animals (Dilzer and Park, 2012). Oils were stripped in advance by the method of Boon et al. (2008) to remove natural antioxidants, then 0.01% tert-butylhydroquinone were added to all diets to minimize lipid oxidation.
Reproduction analysis
One to two day old adult flies were treated with experimental diets for 10 days (Tyler, 2000). Flies were transferred into fresh foods every 24 hours, and the accumulated brood size (egg production numbers) from Day 1 to 10 of treatments were recorded. Hatchability was recorded on day 6. Hatchability was calculated by ratio of larva to egg numbers (Galikova et al., 2015). Length of treatment was based on preliminary results that 0.5% of CLA for 5 days is effective for body composition regulation. The simplified experimental scheme was shown in Fig. 1.
After 5 days of dietary treatments, cuticular lipids were extracted from groups of 12 females with hexane containing 1-docosene as an internal standard, followed by a double hexane rinse (Schal et al., 2001). The resulting extracts were concentrated and analyzed using gas chromatography-mass spectrometry (GC-MS)-QP2010 SE (Shimadzu, Japan) with SUPELCOWAX™ 10 column (100 m, 0.25 mm i.d., 0.25 μm film thickness, Sigma Aldrich, St Louis, MO, USA). The column oven temperature started at 50°C, increased to 200°C at 20°C/min and then to 220°C at 2°C/min and held at 220°C for 162.5 min. The pheromones were identified by comparison of their retention times and quantified using the internal standard and represent as ng per fly.
After removal of cuticular hydrocarbons, flies were homogenized and lipids were extracted by the Folch method (Folch et al., 1957) and transmethylated with 3 N methanolic HCl at 55°C for 40 min to prepare fatty acid methyl esters (FAMEs) (Park et al., 2001). The FAMEs were analyzed using GC-MS with the same method described above. The FAMEs were identified by the National Institute of Standards and Technology (NIST) mass spectrum library and by retention time of the standards. Fatty acid composition was determined from three biological replicates and reported as a percentage of the total identified fatty acids.
Quantitative real-time PCR (qRT-PCR) was conducted according to Sun et al. (2016) with minor modifications. After 5 days of dietary treatments, total RNA was extracted from 8 flies in TRizol reagent, and reverse transcribed to cDNA using a High Capacity cDNA Reverse Transcription Kit. qRT-PCR was performed using TaqMan Gene Expression Master Mix and primers on the StepOnePlus Real-Time PCR System using the following cycling conditions: 1 cycle of 50°C for 2 minutes, 1 cycle of 95°C for 10 minutes, and 40 cycles of 95°C for 15 seconds with 60°C for 1 minute followed by a dissociation curve analysis. Primers of lipid storage droplet-2 (lsd-2, Dm01838905_m1), midway (mdy, Dm01791906_m1), and actin-42A (act42A; a reference gene; Dm02362162_s1) were used.
Larval development
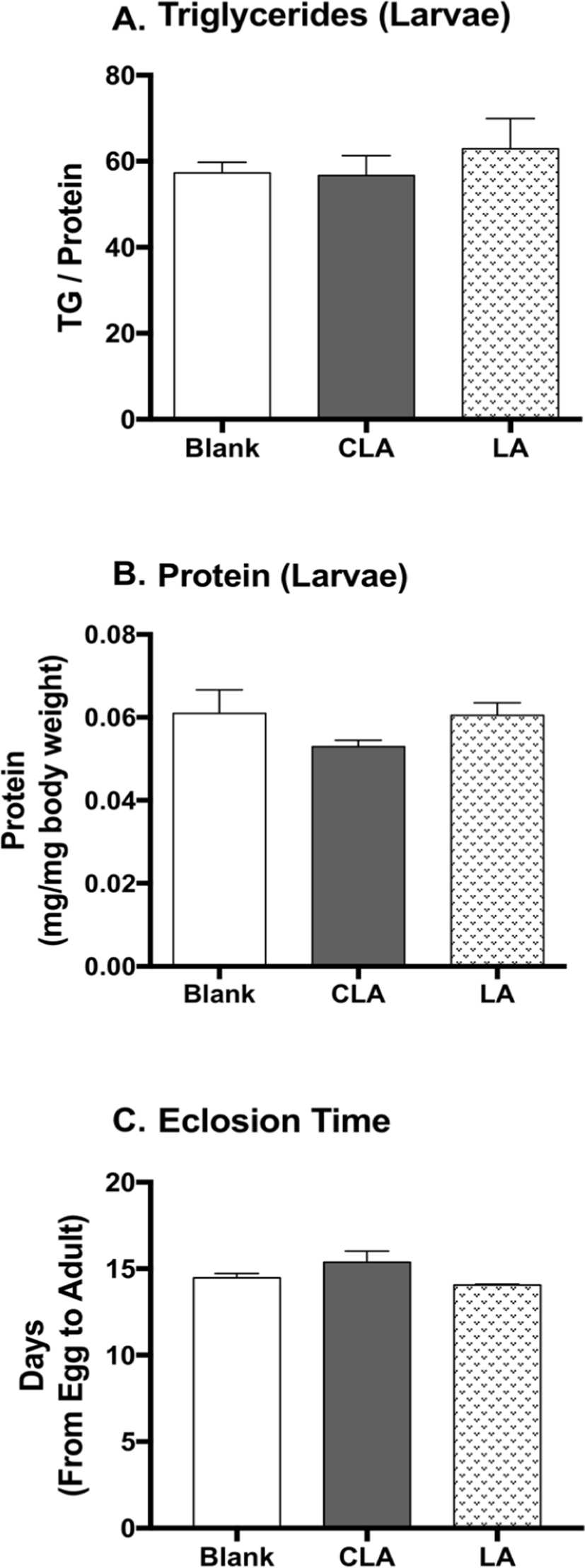
Untreated females laid eggs on fly foods containing 0.5% water, CLA, or safflower oil, then third instar larvae grown from the treatment foods were collected to measure the protein and TG content as body composition. The larvae were rinsed with water, and homogenized with phosphate buffered saline with Tween-20 (PBST), and protein and TG levels were measured using Pierce BCA protein assay kit and Infinity triglycerides reagent, respectively. The protein and TG contents were quantified by the standard curves of bovine serum albumin and glycerol, respectively (Tennessen et al., 2014). Eclosion time was measured as the duration (days) from egg to adult eclosed from pupal cases (Reis, 2016).
Statistical analysis
Data were presented as means with standard error of the mean (SE). Comparisons of various treatments were conducted by the Statistical Analysis System version 9.4 (SAS Institute, Cary, NC) using one-way analysis of variance with post-hoc analyses using Tukey’s test. p-values less than 0.05 were considered statistically significant.
Results
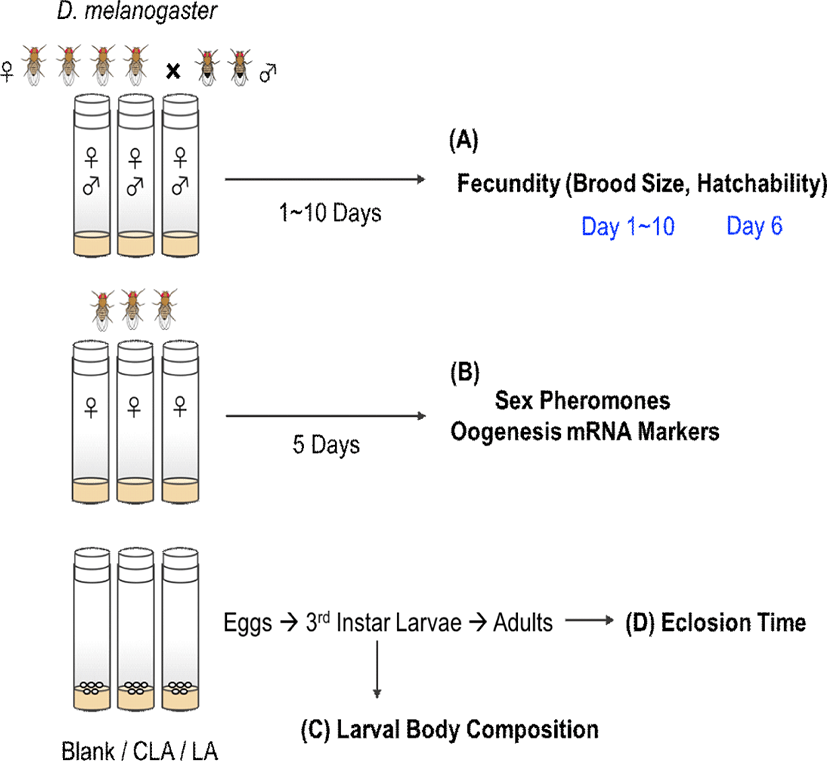
After 5 days of treatments, CLA intake was confirmed by detecting 3.8% of c-9,t-11 and 2.6% of t-10,c-12 CLA isomers in CLA-fed female flies (Table 1). Female flies typically showed peak egg laying behavior on day 4–6 (age of 6–8 days old) (Fig. 2A). However, after 5 days of treatments, the brood sizes were decreased by 32% in LA group and 43% in CLA group (p=0.0027 and 0.0011, respectively) compared with the blank group. Over 10 days of treatment, CLA-fed females had significantly lower accumulated brood sizes by 36% (p=0.0016) and 25% (p=0.0183) compared with the blank group and LA group, respectively. However, there were no significant effects on the hatchability between dietary treatments (Fig. 2B).
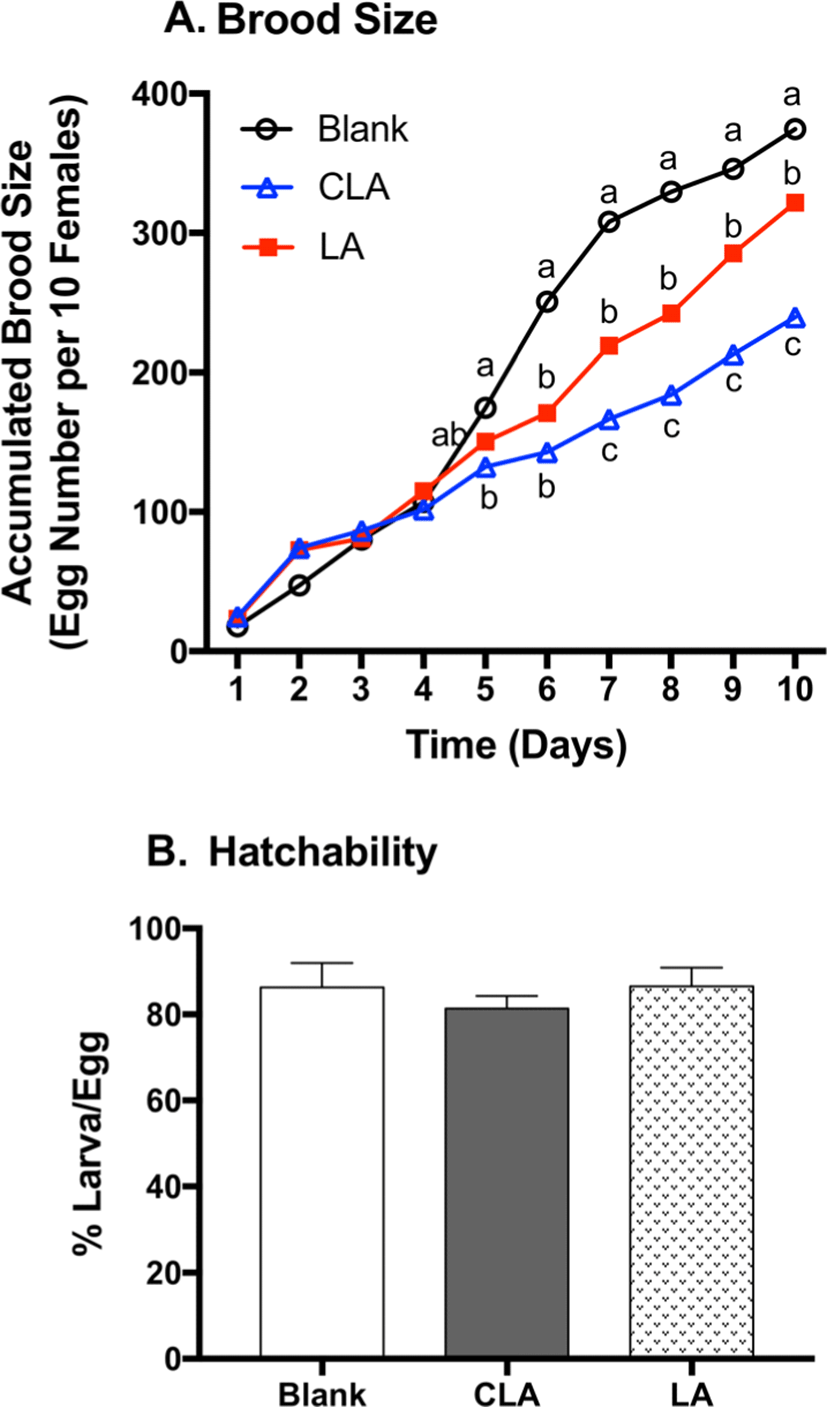
Since 7,11-heptacosadiene (7,11-HD) and 7,11-nonacosadiene (7,11-ND) on female cuticles function as the principle aphrodisiacs to stimulate male courtship and corresponding egg production (Antony et al., 1985), 7,11-HD and 7,11-ND were quantified in female flies (Fig. 3). LA significantly decreased 7,11-HD and 7,11-ND by 43% (p<0.0001) and 41% (p=0.0004) when compared to the respective blanks. CLA significantly decreased 7,11-HD and 7,11-ND by 65% (p<0.0001) and 70% (p<0.0001) when compared to the respective blanks. Moreover, CLA-fed flies lowered 7,11-HD (39%, p=0.0011) and 7,11-ND (50%, p=0.0027) compared to LA groups. As 7,11-HD and 7,11-ND play significant role in courtship stimulation and reproduction behavior, the reduction of 7,11-HD and 7,11-ND by LA and CLA may correspond to the reduction of brood size (Antony et al., 1985).
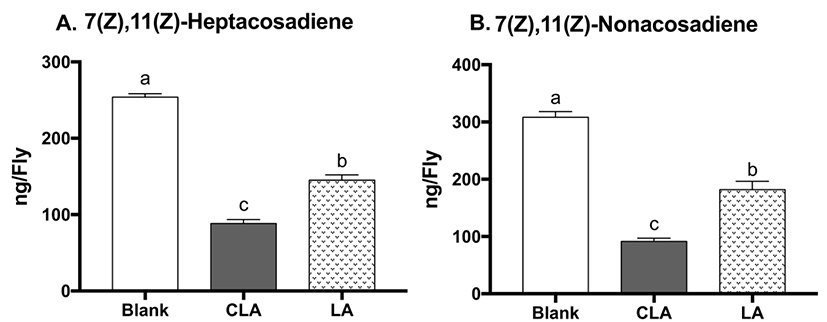
During oogenesis, fly mothers synthesize numerous lipid droplets in the nurse cells of the egg chambers, which are transferred to the oocyte to provide energy for the developing embryo (Kuhnlein, 2012). Lipid storage droplet 2 (lsd2) and midway (mdy) are both particularly abundant in ovaries; lsd2 encodes lipid storage droplet proteins, which regulates transferring lipids to developing oocyte, whereas mdy encodes Drosophila acyl Coenzyme A: diacylglycerol acyltransferase (DGAT), which catalyzes the conversion of diacylglycerol into TG and promotes storage fat deposition within the ovary (Arrese and Soulages, 2010). To evaluate the effects of CLA on oogenesis, mRNA levels of lsd2 and mdy were measured (Fig. 4). CLA showed lower lsd2 gene expression (19% reduction, p=0.0205) compared to LA-treated flies, while there were no differences between CLA and the blank on lsd2. There were no significant changes in mdy gene expression between treatments, suggesting the TG biosynthesis during oogenesis remained unchanged. Altogether, CLA may lower the brood size in part through downregulating lsd2, leading to lower lipid deposition in the eggs in flies.
To study the developmental effects of CLA, larval body composition and the eclosion time were measured (Fig. 5). CLA and LA did not show impacts on larval protein level, triglyceride level, nor eclosion time when compared to the blanks.
Discussion
The current results showed that supplement of CLA decreased fecundity in part through decreasing pheromone in the female adults, without affecting the hatchability and larval development in D. melanogaster. To the best of our knowledge, the current study is the first to determine the effects of CLA on fecundity, pheromone, and oogenesis in this model.
Drosophila encodes two desaturase genes, desat1 and desat2 (Dallerac et al., 2000). The biosynthetic pathway of 7,11-dienes (female sex pheromones) in D. melanogaster generally begins from C16:0 fatty acid (Marcillac et al., 2005); Desat1 introduces a first double bond on the carbon ω-7, then the second double bond on carbon ω-11 is added on elongated mono-unsaturated precursors, C20:1 fatty acid, followed by several elongation steps coupled with a final decarboxylation step to form 7,11-dienes (Marcillac et al., 2005). Previously, it was reported that CLA inhibits delta-9 desaturase (SCD) in mammalian tissues (Smith et al., 2002). However, CLA treated flies showed greater desaturation index (1.61) compared to the LA group (1.48) in the current study (Table 1). Thus, it is unlikely CLA inhibited desaturases that led to reduced 7,11-dienes in this model. As previously reported, CLA reduced total body fat in female flies, which is likely led to reduced 7,11-dienes production due to limited substrate availability, rather than inhibition of desaturases, in this model (Chen et al., 2018).
Previous studies suggested that decreased desaturation indices of fatty acid composition in bird egg yolk by CLA lead to reduced egg production and increased embryo mortality (Aydin and Cook, 2004; Leone et al., 2009). However, there was no effect of CLA on desaturation index nor significant difference in hatchability in the current study. The decreased brood size by CLA may be in part due to the reduction of aphrodisiac pheromones 7,11-HD and 7,11-ND, which are known to promote egg production in female flies. Another study reported that CLA did not change the brood size in C. elegans study (Shen et al., 2018). This inconsistency may be due to the difference in reproductive processes between species; only insect species rely on sex pheromones in the reproduction process. Therefore, the implication of this finding regarding sex pheromone in reproduction to human is relatively limited. More research is needed to determine the working mechanisms of CLA on pheromone biosynthesis pathway.
Lipid metabolism plays an essential role in oogenesis and embryo development. Drosophila DGAT, encoded by the midway (mdy) gene with function in storage lipid deposition from diacylglycerol to TG during oogenesis, is expressed mainly in the fat body and ovaries (Buszczak et al., 2002). In addition, lsd2 is also important in TG accumulation during mid-oogenesis (Teixeira et al., 2003). Overall, CLA attenuated lsd2-associated lipid deposition without influencing mdy gene expression during oogenesis. With limited influence on oogenesis, we did not further examine the effects of CLA on eicosanoids, another key mediator in follicle maturation (Tootle and Spradling, 2008).
Additional factor may be involved in the reproduction changes by CLA. Juvenile hormones, synthesized and secreted by the corpora allata of brain, regulate courtship and mating behaviors in females and males (Bilen et al., 2013). During mating, juvenile hormone is stimulated by sex peptide and further induces onset of pheromone production, including 7,11-dienes, and yolk protein uptake in oogenesis in female flies (Flatt et al., 2005; Moshitzky et al., 1996). In addition, female pheromone profiles and attractiveness are also regulated by insulin and the Target of Rapamycin (TOR) signaling (Kuo et al., 2012). Hence, further studies in these fields would be needed to understand additional effects of CLA on reproduction.
Conclusion
As PUFAs play an essential role in the fecundity, mating behavior, and oogenesis, this study evaluated the effects of CLA on reproduction and development in D. melanogaster. In conclusion, the current study showed that CLA decreased brood size, potentially via reduction of sex pheromone and lipid deposition in oogenesis. Meanwhile, CLA did not alter the hatchability and larval development in D. melanogaster. By understanding the effects of CLA on reproduction and development in D. melanogaster, we will be able to gain better knowledge of the potential role of CLA in development.
